Scroll to:
Monitoring Chicken Infectious Diseases in the Krasnodar Territory and the Republic of Adygea
https://doi.org/10.23947/2949-4826-2025-24-2-55-62
EDN: FNDTPG
Abstract
Introduction. One of the major goals set before the agro-industrial complex is to supply the population of the Russian Federation with the high-quality raw materials and products of animal husbandry. Therefore, poultry farms are implementing modern technologies of intensive poultry farming and carry out regular prophylactic work to ensure epizootological safety of poultry rearing. To make these measures effective, it is necessary to specify the diseases typical for poultry in different regions of the country. The aim of the research is to study and analyse the pathogens isolated from pathological material from chickens reared in the Krasnodar Territory and the Republic of Adygea for planning the effective anti-epizootic, preventive and therapeutic measures.
Materials and Methods. The objects of the study were chickens from the poultry farms of the Krasnodar Territory and the Republic of Adygea and biological material from dead birds (pathological material). In total, 2018 samples were examined during the period of 2019–2023. The research was carried out using a range of methods (epizootological, clinical, pathoanatomical, bacteriological, serological) based on the commonly accepted techniques.
Results. During a five-year research period, 5 pathogens of infectious diseases were isolated from the pathological material obtained from chickens reared in the Krasnodar Territory and the Republic of Adygea (E. coli, E. faecalis, St. aureus, Str. pneumoniaе, S. pullorum), both pure culture and mixed culture. 26 serotypes of E. coli pathogen were isolated from chickens. Whereas, percentage of escherichiosis detected in chickens in the Krasnodar Territory and the Republic of Adygea equaled to 41.43%; enterococcosis — to 26.51%; staphylococcal infection — to 18.11%; streptococcosis — to 12.29 %; salmonellosis — to 0.1%; mixed (escherichiosis and enterococcosis) infection — to 1.64%.
Discussion and Conclusion. Based on the conducted monitoring, it can be concluded that at poultry farms of the Krasnodar Territory and the Republic of Adygea the current situation with the infectious diseases in chickens has improved compared to that five years ago, however, the percentage of escherichiosis, streptococcosis, staphylococcosis and enterococcosis morbidity in poultry is still quite high. Therefore, we recommend the management of the farms to plan the antiepizootic measures aimed at timely prevention of the above mentioned infectious diseases.
Keywords
For citations:
Shevchenko A.A., Konov B.R., Chernykh O.Yu., Shevchenko L.V. Monitoring Chicken Infectious Diseases in the Krasnodar Territory and the Republic of Adygea. Russian Journal of Veterinary Pathology. 2025;24(2):55-62. https://doi.org/10.23947/2949-4826-2025-24-2-55-62. EDN: FNDTPG
Introduction. One of the main objectives of the agro-industrial complex is supplying the population of the Russian Federation with the high-quality raw materials and products of animal husbandry. In the frame of this activity, poultry farms are implementing innovative technologies of intensive poultry farming, as well as carry out regular prophylactic work to ensure epizootological safety of poultry rearing. The most dangerous infectious diseases for chickens are tuberculosis, Newcastle disease, leucosis, escherichiosis, avian influenza, infectious laryngotracheitis, Marek's disease and others. In case of outbreak, these diseases cause mass poultry mortality inducing major economic damage to the poultry industry.
In recent years, the interest of the researchers to animal diseases caused by opportunistic microflora has raised [1][2][3]. In the context of decreased resistance of the organism to infection, violation of veterinary, animal hygiene, sanitary rules for rearing animals, there occur diseases caused by concomitant microflora — streptococcosis, staphylococcosis, escherichiosis, enterococcosis, salmonellosis [4][5]. According to the data obtained by many researchers, agricultural poultry can infect other species of animals, as well as humans, therefore, it is necessary to conduct timely monitoring of microbes and detect pathogenic species to prevent infected products from reaching animals and people [6][7][8]. The use of antibiotics for treatment of sick animals without checking their efficacy against the isolated microorganisms, violation of the dosage and course of therapy lead to rapid adaptation of microbes to unfavourable factors and emergence of the new variants of pathogens, especially of E. coli. Therefore, to prevent infectious diseases, the mandatory action plan of each poultry farm should include quick high-quality diagnostics and various veterinary and sanitary procedures to prevent contamination of animal husbandry raw materials and infecting susceptible animals and people [9][10]. The aim of the article is to study and analyse the pathogens isolated from pathological material from chickens reared in the Krasnodar Territory and the Republic of Adygea to ensure efficient organisation of the anti-epizootic, preventive and therapeutic measures.
Materials and Methods. The research was conducted at the Department of Microbiology, Epizootology and Virology Department, at Territory-level Veterinary Laboratory of Kropotkin and at various poultry farms of the Krasnodar Territory and the Republic of Adygea in the period of 2019–2023. The objects of the study were agricultural poultry (chickens) and isolates of microorganisms (pathological material) from sick and dead birds. A total of 2018 samples were examined. A comprehensive examination was carried out taking into account the epizootological indicators, clinical signs in poultry, pathological changes and the results of laboratory tests as per the isolated pathogen.
Laboratory diagnostics was based on the use of bacteriological tests that enable the isolation of a pathogen, its identification, determination of the type, serotype, pathogenicity of a microorganism [11–14]. The diagnostics was carried out in compliance with the current regulatory documents adopted by the Veterinary Authorities of the Russian Federation on bacteriological methods of examining the pathological material (from the heart, liver, spleen, intestinal contents, bone marrow) obtained from fallen and forcedly killed birds.
The study on the isolation of the pathogen of colibacillosis (escherichiosis) was carried out in compliance with the “Methodological Guidelines for the Bacteriological Diagnostics of Colibacillosis (Escherichiosis) in Animals”, approved on July 27, 2000 by the Deputy Head of the Veterinary Medicine Department of the Ministry of Agriculture and Food of the Russian Federation.
The study on enterococcosis and mixed intestinal infection was carried out in compliance with the “Methodological Guidelines for the Bacteriological Diagnostics of Mixed Intestinal Infection in Young Animals Caused by Pathogenic Enterobacteria” No. 13-7-2/1759 of October 11, 1999 adopted by the Veterinary Medicine Department of the Ministry of Agriculture and Food of the Russian Federation.
The study on staphylococcosis was carried out in compliance with the “Methodological Guidelines for Laboratory Diagnostics of Staphylococcosis in Animals” approved on June 30, 1987 by the Head of the Main Veterinary Directorate of the State Agro-Industrial Committee of the USSR.
The study on the isolation of pathogens of streptococcosis was carried out in compliance with the “Methodological Guidelines for Laboratory Diagnostics of Streptococcosis in Animals” approved on September 25, 1990 by the Deputy Head of the Main Veterinary Directorate with the State Veterinary Inspectorate under the State Commission of the USSR Council of Ministers for Food and Procurement.
For the study on salmonellosis, “Methodological Guidelines MU 4.2.2723–10 for Laboratory Diagnostics of Salmonellosis, Detection of Salmonella in Food Products and Environmental Objects” published by the Federal Centre for Hygiene and Epidemiology of Rospotrebnadzor in 2011 was used.
Standard laboratory equipment was used to conduct the research: a thermostat at 37 °C, and a microscope (“Biolam”, Russia) [15][16]. Bacteriological tests included determination of the morphological, tinctorial, bacteria culture, and serological indicators in the isolated microorganisms [17–20].
The epizootic situation was assessed based on the analysis of disease outbreaks registered in the record books of the farms.
Research Results. During laboratory study of the pathological material from poultry from the various farms of the Krasnodar Territory and the Republic of Adygea in the period of 2019–2023, 5 pathogens of infectious diseases were isolated: escherichiosis (E. coli), enterococcosis (E. faecalis), staphylococcosis (St. aureus), streptococcosis (Str. pneumoniae), mixed microflora (E. coli, E. faecalis), salmonellosis (S. pullorum) (Fig. 1, Table 1).
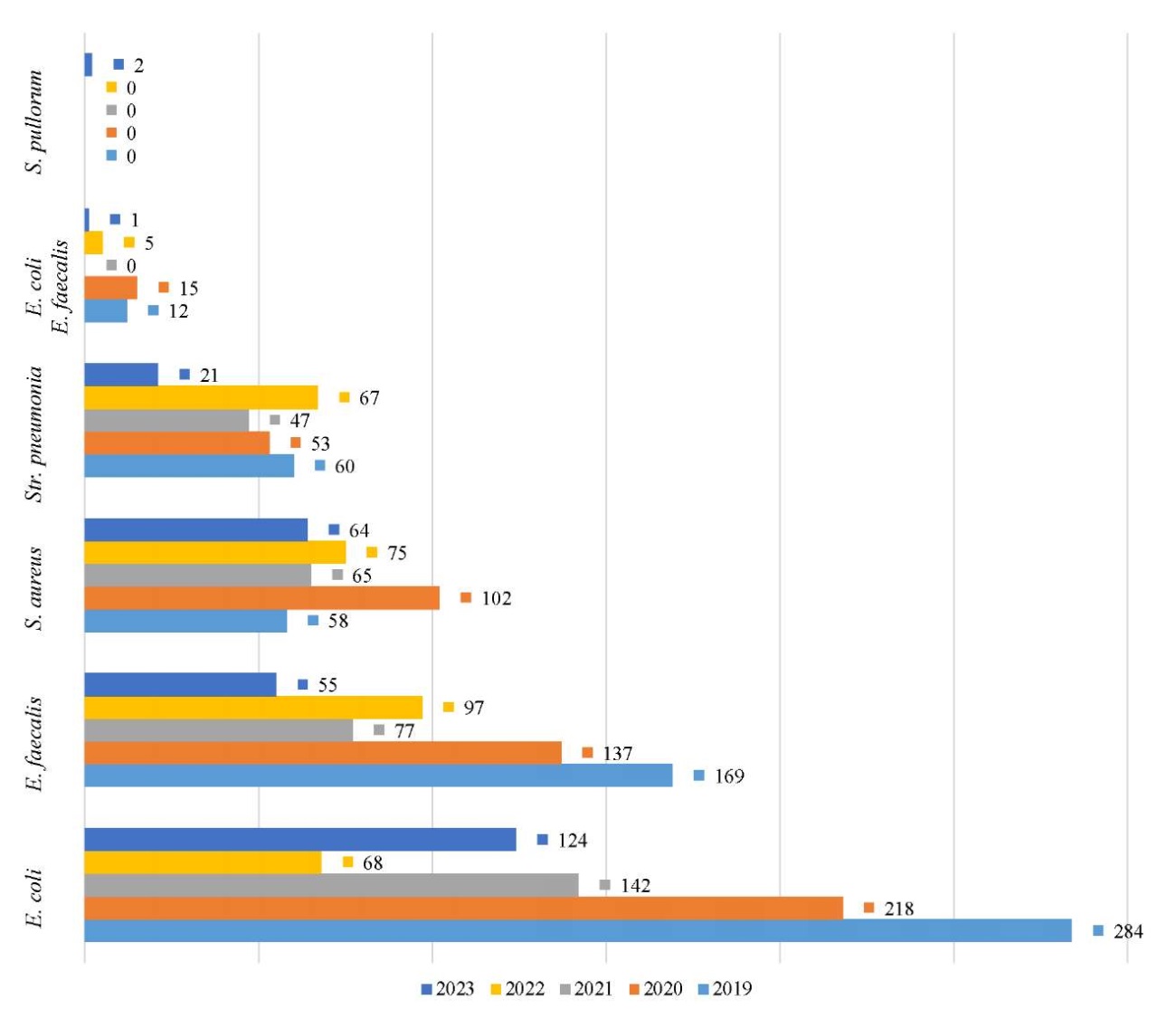
Fig. 1. Dynamics of pathogen isolation from chickens reared in the Krasnodar Territory and the Republic of Adygea in the period of 2019–2023
Table 1
Monitoring cases of infectious diseases in chickens in the Krasnodar Territory and the Republic of Adygea in the period of 2019–2023
|
No |
Title |
2019 |
2020 |
2021 |
2022 |
2023 |
Total |
||||||
|
number |
%* |
number |
%* |
number |
%* |
number |
%* |
number |
%* |
number |
%* |
||
|
1. |
Escherichiosis |
284 |
48,54 |
218 |
41,52 |
142 |
42,90 |
68 |
21,79 |
124 |
46,79 |
836 |
41,43 |
|
2. |
Enterococcosis |
169 |
28,88 |
137 |
26,09 |
77 |
23,26 |
97 |
31,08 |
55 |
20,75 |
535 |
26,51 |
|
3. |
Staphylococcosis |
58 |
9,91 |
102 |
19,42 |
65 |
19,63 |
75 |
24,03 |
64 |
24,15 |
364 |
18,04 |
|
4. |
Streptococcosis |
60 |
10,25 |
53 |
10,09 |
47 |
14,19 |
67 |
21,47 |
21 |
7,92 |
248 |
12,29 |
|
5. |
Escherichiosis + enterococcosis |
12 |
2,05 |
15 |
2,85 |
0 |
0 |
5 |
1,6 |
1 |
0,37 |
33 |
1,64 |
|
6 |
Salmonellosis |
2 |
0,34 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0,1 |
|
Total |
585 |
18,28 |
525 |
26,01 |
331 |
16,40 |
312 |
15,46 |
265 |
13,13 |
2018 |
100,0 |
|
Note: * — percentage of the total number of samples tested (n=2018)
A significant number of different E. coli serotypes are known. Intestinal bacterium (E. coli) has different types of antigens: O-antigen (somatic), K-antigen (capsular), H-antigen (flagellar), some of them are pathogenic, others are non-pathogenic. Of the pathogenic E. coli from chickens, 26 serotypes of somatic O-antigen were isolated, the most common were serotypes 1, 2, 9, 15, 20, 27, 33, 45, 55, 78, 139 (Fig. 2, Table 2).
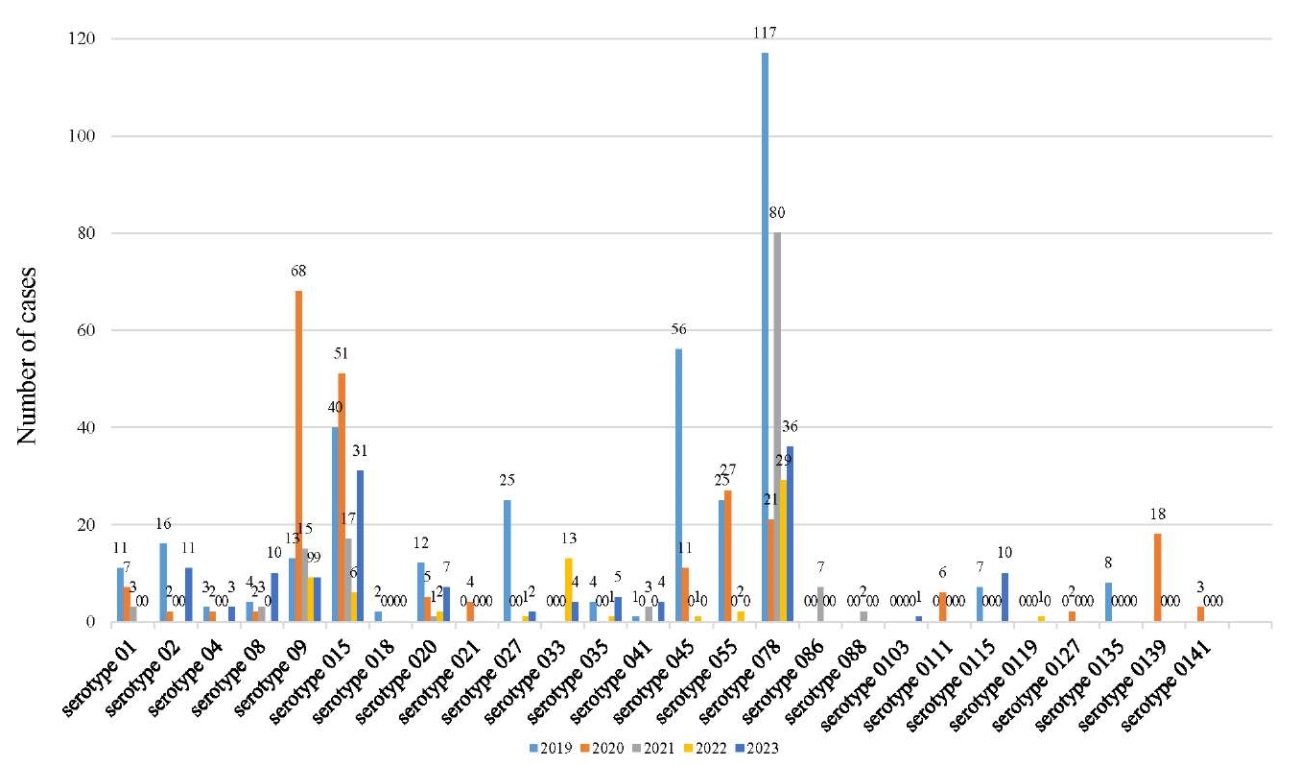
Fig. 2. Dynamics of isolated E. coli serotypes from pathological material from chickens reared in the Krasnodar Territory and the Republic of Adygea in the period of 2019–2023.
Table 2
Isolated E. coli serotypes from pathological material from chickens reared in the Krasnodar Territory and the Republic of Adygea in the period of 2019–2023
|
E. coli Serotypes |
2019 |
2020 |
2021 |
2022 |
2023 |
Total |
|||||||
|
Number |
%* |
Number |
%** |
Number |
%*** |
Number |
%**** |
Number |
%***** |
Number |
% |
||
|
1. |
01 |
11 |
3,87 |
7 |
3,21 |
3 |
2,01 |
0 |
0 |
0 |
0 |
21 |
2,22 |
|
2. |
02 |
16 |
5,63 |
2 |
0,91 |
0 |
0 |
0 |
0 |
11 |
9,01 |
18 |
1,9 |
|
3. |
04 |
2 |
0,70 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
2,45 |
10 |
1,05 |
|
4. |
08 |
4 |
1,40 |
0 |
0 |
3 |
2,01 |
0 |
0 |
10 |
8,19 |
22 |
2,32 |
|
5. |
09 |
13 |
4,57 |
68 |
31,19 |
15 |
10,06 |
9 |
13,23 |
9 |
7,37 |
114 |
12,05 |
|
6. |
015 |
40 |
14,08 |
51 |
23,39 |
17 |
11,40 |
6 |
8,82 |
31 |
25,40 |
145 |
15,32 |
|
7. |
018 |
2 |
0,70 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0,21 |
|
8. |
020 |
12 |
4,22 |
5 |
2,29 |
1 |
0,67 |
2 |
2,94 |
7 |
5,73 |
27 |
2,85 |
|
9. |
021 |
0 |
0 |
4 |
1,83 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0,42 |
|
10. |
027 |
5 |
1,76 |
0 |
0 |
0 |
0 |
1 |
1,47 |
2 |
1,63 |
28 |
2,96 |
|
11. |
033 |
0 |
0 |
0 |
0 |
0 |
0 |
13 |
19,11 |
4 |
3,27 |
17 |
1,8 |
|
12. |
035 |
4 |
1,40 |
0 |
0 |
0 |
0 |
1 |
1,47 |
0 |
0 |
5 |
0,53 |
|
13. |
041 |
1 |
0,35 |
0 |
0 |
0 |
0 |
3 |
4,41 |
0 |
0 |
4 |
0,42 |
|
14. |
045 |
26 |
9,15 |
0 |
0 |
11 |
7,38 |
1 |
1,47 |
0 |
0 |
68 |
7,19 |
|
15. |
055 |
25 |
8,80 |
27 |
12,38 |
0 |
0 |
2 |
2,94 |
0 |
0 |
54 |
5,7 |
|
16. |
078 |
115 |
40,49 |
21 |
9,63 |
80 |
53,69 |
29 |
42,64 |
36 |
29,50 |
342 |
36,15 |
|
17. |
086 |
0 |
0 |
0 |
0 |
7 |
4,69 |
0 |
0 |
0 |
0 |
7 |
0,73 |
|
18. |
088 |
0 |
0 |
0 |
0 |
2 |
1,34 |
0 |
0 |
0 |
0 |
2 |
0,21 |
|
19. |
0103 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0,81 |
1 |
0,10 |
|
20. |
0111 |
0 |
0 |
6 |
2,75 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
0,63 |
|
21. |
0115 |
0 |
0 |
7 |
3,21 |
0 |
0 |
0 |
0 |
10 |
8,19 |
17 |
1,8 |
|
22. |
0119 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1,47 |
0 |
0 |
1 |
0,10 |
|
23. |
0127 |
0 |
0 |
2 |
0,91 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0,21 |
|
24. |
0135 |
8 |
2,81 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
0,84 |
|
25. |
0139 |
0 |
0 |
18 |
8,25 |
0 |
0 |
0 |
0 |
0 |
0 |
18 |
1,9 |
|
26. |
0141 |
0 |
0 |
0 |
0 |
3 |
2,01 |
0 |
0 |
0 |
0 |
3 |
0,31 |
|
Total |
284 |
30,02 |
218 |
23,04 |
149 |
15,75 |
68 |
7,18 |
122 |
12,89 |
946 |
100 |
|
Note: * — percentage of the total number of cases of escherichiosis in 2019 (n=284)
** — percentage of the total number of cases of escherichiosis in 2020 (n=218)
*** — percentage of the total number of cases of escherichiosis 2021 (n=149)
**** — percentage of the total number of cases of escherichiosis in 2022 (n=68)
***** — percentage of the total number of cases of escherichiosis in 2023 (n=122)
The pathogenic microorganisms E. coli, E. faecalis, St. aureus, Str. pneumoniaе, S. pullorum detected as a result of monitoring, both pure culture and mixed culture with other microbes, caused the following infectious diseases in chickens: escherichiosis (41.43%), enterococcosis (26.51%), staphylococcosis (18.04%), streptococcosis (12.29%), mixed intestinal infection (escherichiosis and enterococcosis) — 1.64%, salmonellosis (0.1%).
Discussion and Conclusion. Based on the results of the conducted monitoring, a tendency towards improvement of the epizootic situation at poultry farms of the Krasnodar Territory and the Republic of Adygea can be noted compared to the situation five years ago. Nevertheless, the percentage of chickens infected with escherichiosis, streptococcosis, staphylococcosis and enterococcosis remains quite high. Due to high variability of the pathogen causing escherichiosis (E. Coli) (26 serotypes have been isolated over the past 5 years), it can infect humans through raw materials and products of animal husbandry. In this regard, the management of poultry farms is strongly recommended to take timely measures on diagnostics and prophylaxis of the above infectious diseases, as well as to treat poultry located in the unfavorable places after mandatory determination of the isolated pathogenic microorganisms’ sensitivity to antibiotics. At the same time, the biological features of these types of microbes must be taken into account when developing the efficient technology for rearing poultry and animals at poultry and husbandry farms.
References
1. Asmolova OL, Mandro NM, Litvinova ZA. Broilers' Susceptibility to Bacteria Isolated from Synanthropic Birds. Far Eastern Agrarian Journal. 2017;(2(42)):92–97.
2. Verevkina MN. Salmonellosis of Birds. In: Proceedings of the 75th Scientific and Practical Conference “Diagnosis, Treatment and Prevention of Diseases in Farm Animals”. Stavropol: Stavropol State Agrarian University; 2010. P. 9–10.
3. Davleev AD. Prevention of Salmonellosis in European Poultry Farming. Tsenovik. Agricultural Review. 2020;9:6–9.
4. Dmitrieva ME. Veterinary Well-Being in Industrial Poultry Production. Animal Husbandry of Russia. 2016;S1:46–48.
5. Dobrina MN. The Role of Pigeons in the Spread of Salmonella Enteritidis Infection of Birds in Poultry Farms. Veterinaria i kormlenie (Veterinary Medicine and Feeding). 2011;3:22–23.
6. Corella H.S. Avian Salmonellosis and Prospects for Its Control. BIO. 2018;6(213):10–11.
7. Kostenko YuG, Chramov MV, Davleev AD. Problem of Food Salmonellosis and Ways for It Decision. Veterinariya (Veterinary Medicine). 2016;2:9–12.
8. Litvinova ZA, Mandro NM, Punina PV. The Spreading of Bacterial Infectious Diseases of the Poultry in the Upper Amur Region. Bulletin of KSAU. 2020;4(157):102–106. http://doi.org/10.36718/1819-4036-2020-4-102-106
9. Lenchenko EM, Khay PhV, Vatnikov YuA, Medvedev IN, Gavrilov VA. Etiological Structure and Differential Diagnostics of Salmonellosis of Birds. RUDN Journal of Agronomy and Animal Industries. 2017;12(4):359–367.
10. Musiev DG, Tsakhaeva RO, Azaev GKh, Gunashev ShA, Abduragimova RM, Mayorova TL, et al. Laboratory Diagnostics of Bird Salmonellosis. Daghestan GAU Proceedings. 2020;2(6):72–75.
11. Novikova OB, Pavlova M. Actual and New Diseases of Birds of Bacterial Etiology. Actual Issues in Agricultural Biology. 2017;4(6):40–44.
12. Novikova OB, Nikitina NV, Pavlova MA, Karpova OS, Bartenev AA. Control and Prevention of Bacterial Diseases in the Waterfowl. Ptitsevodstvo (Poultry Farming). 2019;(11–12):93–99. http://doi.org/10.33845/0033-3239-2019-68-11-12-93-99
13. Punina PV. Spread of Infectious Diseases of Birds in the Russian Federation. In: Proceedings of the XIX Regional Scientific and Practical Conference “Youth of the XXI Century: A Step into the Future”. Blagoveshchensk: Far Eastern State Agrarian University. 2018. P. 57–58.
14. Palomatskova NA. Diagnosis of Chicken Salmonellosis by the Enzyme Immunoassay. Veterinary Pathology. 2011;(4):51–54.
15. Shevchenko АА, Chernykh OYu, Manakova AYu, Klimenko AA, Lazaridi DG, Marchenko VV. Results of Monitoring of Microorganisms in Animals in the Krasnodar Krai. Science Review. 2024;19(6(138)):1097–1106.
16. Tatarnikova NA, Chugunova EO. Circulation of Various Salmonellas Serotypes in Population of Animals and Birds in Perm Region. Veterinariya (Veterinary medicine). 2016;(2):26–30.
17. Chugunova EO, Tatarnikova NA, Prokhorova TS, Maul OG. Contamination Salmonellae of Production f Poultry Farming. Modern Problems of Science and Education. 2014;(6):1823. URL: https://www.scienceeducation.ru/ru/article/view?id=15850 (accessed: 12.03.2025).
18. Chugunova EO. Cultural Properties of Salmonella Spp. Enriched by Innovation Method. Bulletin of KSAU. 2016;(5(116)):72–77.
19. Shevchenko AA, Toropyno AV, Shevchenko LV. Biological Research in Escherichiosis. Proceedings of the Kuban State Agrarian University. 2018;71:97–102. https://doi.org/10.21515/1999-1703-71-97-102
20. Gast RK, Guraya R, Jones DR, Anderson KE, Karcher DM. Colonization of Internal Organs by Salmonella Enteritidis in Experimentally Infected Laying Hens Housed in Enriched Colony Cages at Different Stocking Densities. Poultry Science. 2016;95(6):1363–1369. https://doi.org/10.3382/ps/pey541
About the Authors
A. A. ShevchenkoRussian Federation
Alexander A. Shevchenko - Dr.Sci. (Veterinary Sciences), Professor, Head of the Microbiology, Epizootology and Virology Department.
13, Kalinina Str., Krasnodar, 350044
B. R. Konov
Russian Federation
Bayzet R. Konov - Postgraduate Degree Student.
13, Kalinina Str., Krasnodar, 350044
O. Yu. Chernykh
Russian Federation
Oleg Yu. Chernykh - Dr.Sci (Veterinary Sciences), Professor, Director of Territory-level Veterinary Laboratory of Kropotkin, Leading Research Associate of the North Caucasus Research Veterinary Institute, Branch of the Federal Rostov Agrarian Scientific Center.
303, Krasnoarmeyskaya Str., Kropotkin, Krasnodar Territory, 352380; 1, Institutskaya Str., Rassvet Settlement, Aksay District, Rostov Region, 346735
L. V. Shevchenko
Russian Federation
Lyudmila V. Shevchenko - Dr.Sci. (Veterinary Sciences), Associate Professor, Senior Research Associate.
1, Institutskaya Str., Rassvet Settlement, Aksay District, Rostov Region, 346735
The study identified the pathogens of infectious diseases in chickens in the Krasnodar Territory and the Republic of Adygea. The most widespread were colibacillosis and enterococcosis, detected in 41.43% and 26.51% of cases, respectively. 26 serotypes of E. coli were isolated, which proves its high variability. The monitoring revealed a tendency towards improvement of the epizootic situation in the region. It is recommended to carry out preventive measures and organise treatment based on diagnostics of sensitivity to antibiotics.
Review
For citations:
Shevchenko A.A., Konov B.R., Chernykh O.Yu., Shevchenko L.V. Monitoring Chicken Infectious Diseases in the Krasnodar Territory and the Republic of Adygea. Russian Journal of Veterinary Pathology. 2025;24(2):55-62. https://doi.org/10.23947/2949-4826-2025-24-2-55-62. EDN: FNDTPG


























