Scroll to:
H₂S-Dependent Mechanisms of Caspase-3 Expression and Localization in Brain Cells of Mice with Traumatic Brain Injury
https://doi.org/10.23947/2949-4826-2025-24-2-19-28
EDN: HPMRMX
Abstract
Introduction. Traumatic brain injury (TBI) is a neurotrauma widespread in animals. TBI causes a complex cascade of pathological processes: primary brain injury turns into secondary brain injury associated with inflammation, oxidative stress, excitotoxicity and apoptosis. Secondary injury aggravates the condition after injury. In this regard, the role of hydrogen sulfide (H₂S) as a gasotransmitter involved in neuromodulation, anti-inflammatory, antioxidant and anti-apoptotic processes in the central nervous system is of particular interest. Caspase-3 is an important element in TBI-induced apoptosis. H₂S has a potential to modulate the expression and activity of caspase-3 affecting the survival of nerve cells and brain recovery after TBI. However, H₂S-dependent mechanisms of caspase-3 regulation in traumatic injury are not fully investigated. The aim of the research is to study the role of H₂S in the expression and localization of caspase-3 in neurons and astrocytes of mice with TBI.
Materials and Methods. The research was conducted at the Bioengineering Department of DSTU (Rostov-on-Don) from April 20 to June 1, 2024 in conditions compliant with the international and national standards. The objects of the study were 36 adult male mice divided into three groups: control group and two experimental ones. TBI was simulated by dropping a weight (200 g) on the intact skulls of mice anesthetized with chloral hydrate. During 7 days after the TBI, the animals were daily administered the sodium sulfide (Na₂S), a donor of H₂S, which can efficiently release H₂S, or the aminooxyacetic acid (AOAA), an inhibitor of cystathionine β-synthase (CBS), an enzyme responsible for the endogenous synthesis of H₂S, until the animals were withdrawn from the experiment. The use of Na₂S and AOAA enabled efficient modulation of the level of endogenous H₂S in the brain. The control group was administered physiological saline solution. Brain sections fixed in 4% paraformaldehyde (PFA) solution were incubated with antibodies to caspase-3 and to the neuronal nuclear antigen (NeuN) or to the astrocytic marker (GFAP). Colocalization was assessed using the ImageJ software. Caspase-3 expression in the brain penumbra was analysed by Western blotting using primary antibodies against caspase-3 and β-actin and secondary antibodies IgG conjugated to horseradish peroxidase. For detection, the chemiluminescence method was used.
Results. The initial level of caspase-3 in the brain cells of mice under study was low. Seven days after injury, TBI had induced caspase-3 expression in neurons and glial cells of the ipsilateral injured hemisphere in animals of all groups. Administering the donor Na₂S led to decrease of caspase-3 level in neurons by 32%, whereas administering the inhibitor AOAA led to its increase by 31% compared to the injured nerve cells of animals from the control group, which were administered the physiological saline solution. This was confirmed by the values of the M1 colocalization coefficient demonstrating colocalization of caspase-3-positive cells with the neuronal nuclear antigen (NeuN). Similar effects were demonstrated in astrocytes, which were visualized using the astrocyte-specific marker GFAP. Western blot analysis confirmed these data and showed a significant decrease of caspase-3 level with administering Na₂S and its increase with that of AOAA 7 days after TBI.
Discussion and Conclusion. The results of the study demonstrate that TBI leads to significant activation of caspase-3 in neurons and astrocytes of the injured hemisphere of mice brain, which means development of apoptosis in response to traumatic injury. Administering Na₂S has efficiently decreased caspase-3 level, which indicates its neuroprotective and anti-apoptotic effect. Whereas, administering the AOAA has induced an increase in caspase-3 expression, which confirms the important role of CBS and, therefore, of H₂S in regulation of cell death after TBI. The reliability of these findings was ascertained by both immunohistochemical and Western blot analysis. The obtained data contribute to better understanding the fundamental H₂S-dependent signaling mechanisms of survival and death of neurons and glial cells during traumatic injury of the nervous system. The CBS inhibitor and H₂S donor used in our study may serve a basis for development of the clinically efficient neuroprotective agents.
Keywords
For citations:
Rodkin S.V., Kirichenko E.Yu. H₂S-Dependent Mechanisms of Caspase-3 Expression and Localization in Brain Cells of Mice with Traumatic Brain Injury. Russian Journal of Veterinary Pathology. 2025;24(2):19-28. https://doi.org/10.23947/2949-4826-2025-24-2-19-28. EDN: HPMRMX
Introduction. Traumatic brain injury (TBI) is one of the most widespread causes of deaths and disabilities in animals. TBI leads to a complex cascade of pathophysiological processes, including primary mechanical injury of brain tissue and subsequent secondary injury due to inflammation, oxidative stress, excitotoxicity and apoptosis. Secondary injury developing after trauma significantly affects the progression of the severity of condition, therefore, scientists worldwide stive to find efficient treatment strategies and neuroprotective agents [1–3].
In recent years, the role of gasotransmitters, such as hydrogen sulfide (H₂S), in the pathophysiology of TBI is becoming an object of growing interest. For a long time H₂S was deemed to be just a toxic gas but now it is recognized as an important bioactive molecule capable of participating in a number of physiological and pathological processes, including neuromodulation, vascular regulation, anti-inflammatory activity and antioxidant protection. In the central nervous system, H₂S acts as a neuromodulator and neuroprotector, affecting various signaling pathways, including pathways of apoptotic cell death [4][5].
One of the key pro-apoptotic proteins is caspase-3 — a cysteine protease that cleaves proteins specifically after aspartate and is an important effector of apoptosis. It has been demonstrated that caspase-3 can be a potential target for H₂S and its active derivatives. However, information about the role of H₂S-dependent mechanisms in the regulation of caspase-3 is contradictory. Some studies show that H₂S can decrease the expression of this enzyme [6–8], while other scientific papers demonstrate H₂S-dependent expression of caspase-3 and a cytotoxic effect [9–11]. Thus, the mechanisms enabling H₂S to affect the expression and localization of caspase-3 in TBI are still poorly understood [9].
The research aims to investigate H₂S-dependent mechanisms of caspase-3 regulation during TBI. We expect H₂S to be capable of modulating caspase-3 expression and localization, thereby affecting the intensity and duration of the neuroinflammatory response during TBI. Study of these mechanisms can contribute to better understanding the complex pathogenesis of TBI and open new perspectives for developing therapeutic strategies aimed at modulating neuroinflammation and improving recovery processes in the brain.
Materials and Methods
Ethical Approval
All studies were approved by the DSTU Ethics Committee and were held in compliance with the international bioethics requirements.
Object of Research and Procedures
The research was conducted in the “MedTsifra” laboratory of the Bioengineering Department, Bioengineering and Veterinary Medicine Faculty of DSTU, from April 20 to June 1, 2024. In total, 36 adult male CD-1 mice aged 14 to 15 weeks and weighing 20–25 g were included in the experiment. The mice were kept under standard vivarium conditions: in plastic cages with sawdust bedding, at a temperature of 22 ± 2 °C, relative humidity of 50–60%, and a 12-hour light-dark regime. They were fed standard granulated feed for laboratory rodents, with free access to water.
The mice were divided into three groups: the control group, which was injected with physiological saline solution, and two experimental groups, which were injected with the sodium sulfide (Na₂S, 0.1 mg/kg; Khimikon, Russia) — the donor of H₂S, or the aminooxyacetic acid (AOAA, 5.0 mg/kg; Tianjin Xidian Chemical Technology Co., Ltd., China) — the inhibitor of cystathionine-β-synthase (CBS), respectively. The use of Na₂S and AOAA made it possible to efficiently modulate the level of endogenous H₂S in the brain: Na₂S is capable of efficient release of H₂S; and AOAA is an inhibitor of CBS, the enzyme responsible for the endogenous synthesis of H₂S. After injury, the drugs were administered intraperitoneally daily during 7 days until the animals were withdrawn from the experiment.
One and the same version of the standard protocol for simulating TBI was used for all groups. Mice were anesthetized by intraperitoneal injection of chloral hydrate (300 mg/kg). A weight was then dropped from a height of 3 cm on the intact skull. The coordinates of the weight drop were set as follows: 2 mm dorsal to bregma, 1 mm lateral to the midline. A weight-drop device consisting of a metal rod (with a tip of 3 mm in diameter, 5 mm in length) weighing 200 g was used to perform a stroke. After awakening from anesthesia, the mice returned to their usual place of staying [12].
Immunofluorescence Analysis
The following protocol was used to identify caspase-3 localization 7 days after TBI. The brain area around the focus of necrosis resulting from the stroke by a weight and the area from the left undamaged hemisphere were excised. The excised piece of cerebral cortex of a mouse was fixed in 4% PFA for 12 hours at +4°C with constant stirring. Then, 20-μm-thick sections were obtained using a Leica VT1000 S automatic vibrating blade microtome (Leica Biosystems Nussloch, Germany). To ensure background blocking, the sections were incubated for 60 min at +24°C in a 5% BSA and 0.3% Triton X-100 solution.
Then the sections were incubated with primary antibodies anti-CASP3 (1:100, Rabbit, AF6311, Affinity, China, antibody against caspase-3) and anti-NeuN (1:1000, Mouse, FNab10266, Fine Test, China, antibody against the neuronal nuclear antigen NeuN) for 48 hours at +4 °C. After repeated washing in PBS, the sections were incubated with secondary antibodies anti-Rabbit IgG (H+L) Fluor 488-conjugated (1:500; S0018, Affinity Biosciences, China) and anti-Mouse IgG (H+L) Fluor 594-conjugated (1:500; S0005, Affinity Biosciences, China). The sections were then immersed into glycerol and used as the specimens for analysis carried out by means of the Altami LUM 1 Fluorescent Microscope (Ningbo Haishu Honyu Opto-Electro Co., LTD, China in collaboration with “Altami” Co., Russia) equipped with a high-resolution digital camera EXCCD01400KPA (Hangzhou Toup Tek Photonics Co., Ltd., China).
The analysis of colocalization of caspase-3 and NeuN was carried out using a special Image J software package supplemented with the JACoP plugin [13]. Mean Fluorescence Intensity of caspase-3 was evaluated based on 10 photographs of sections for each mouse (from experimental and control groups) according to the following formula:
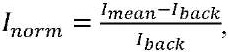
where Imean is the mean intensity in the studied area, Iback is the mean background intensity.
Immunoblotting (Western blotting)
The expression of caspase-3 in conditions of activation or inhibition of the H₂S signaling pathway in the penumbra of the cerebral cortex of mice after TBI was studied using the method of Western blotting. Seven days after injury, the animals were decapitated, the brain was removed, and the infarcted area was excised on ice using a hollow cutter. Next, a 2-mm-wide ring corresponding to the penumbra area was excised using another cutter. These rings were compared with control cortex specimens taken from the other side of brain of the same mouse. Specimens were homogenized using the Tissue Lysis Buffer solution with protease and phosphatase inhibitors added. The resulting homogenate was then centrifuged to obtain a supernatant. The protein concentration in the supernatant was determined using the Bradford method. Then, 25 μg of protein in 20 μl were added to the wells in a polyacrylamide gel and subjected to electrophoresis in the presence of sodium dodecyl sulfate. Mini-PROTEAN Tetra (Bio-Rad, USA) was the equipment used. After electrophoresis, proteins were transferred to a nitrocellulose membrane by the semi-dry transfer using the Trans-Blot Turbo Transfer System (Bio-Rad, USA). Then, primary antibodies against caspase-3 (1:100, AF6311, Affinity, China) and against β-actin and secondary antibody anti-Rabbit IgG conjugated to horseradish peroxidase (HRP) (1:1000; S0001, Affinity Biosciences, China) were used. Then, chemiluminescent protein detection was carried out. Chemiluminescence was detected using the SH-Advance523 gel documentation system (Shenhua Science Technology Co., Ltd., China).
Statistical analysis
For statistical processing and analysis of the obtained results, the method of single-factor analysis of variance was used. Pairwise multiple comparisons were made using the Tukey's Honest Significant Difference (HSD). The parametric tests were used if the rules of normality and homogeneity of variances were met, which were assessed by the Shapiro-Wilk and Brown-Forsythe tests, respectively. If the assumption about normality and homogeneity of variances was not confirmed, the nonparametric Kruskal-Wallis test was used. Statistically significant reliability was considered at p<0.05. The SigmaPlot software package, version 12.5 (Systat Software, Inc., USA), was used for statistical analysis.
Results. Immunofluorescence microscopy demonstrated that caspase-3 localized in nerve and glial cells. Their nucleoplasm was visualized using the fluorochrome Hoechst 33342. Also, the analysis revealed colocalization of caspase-3 and NeuN — a neuronal nuclear antigen, and GFAP — an astrocyte marker (Fig. 1 a).
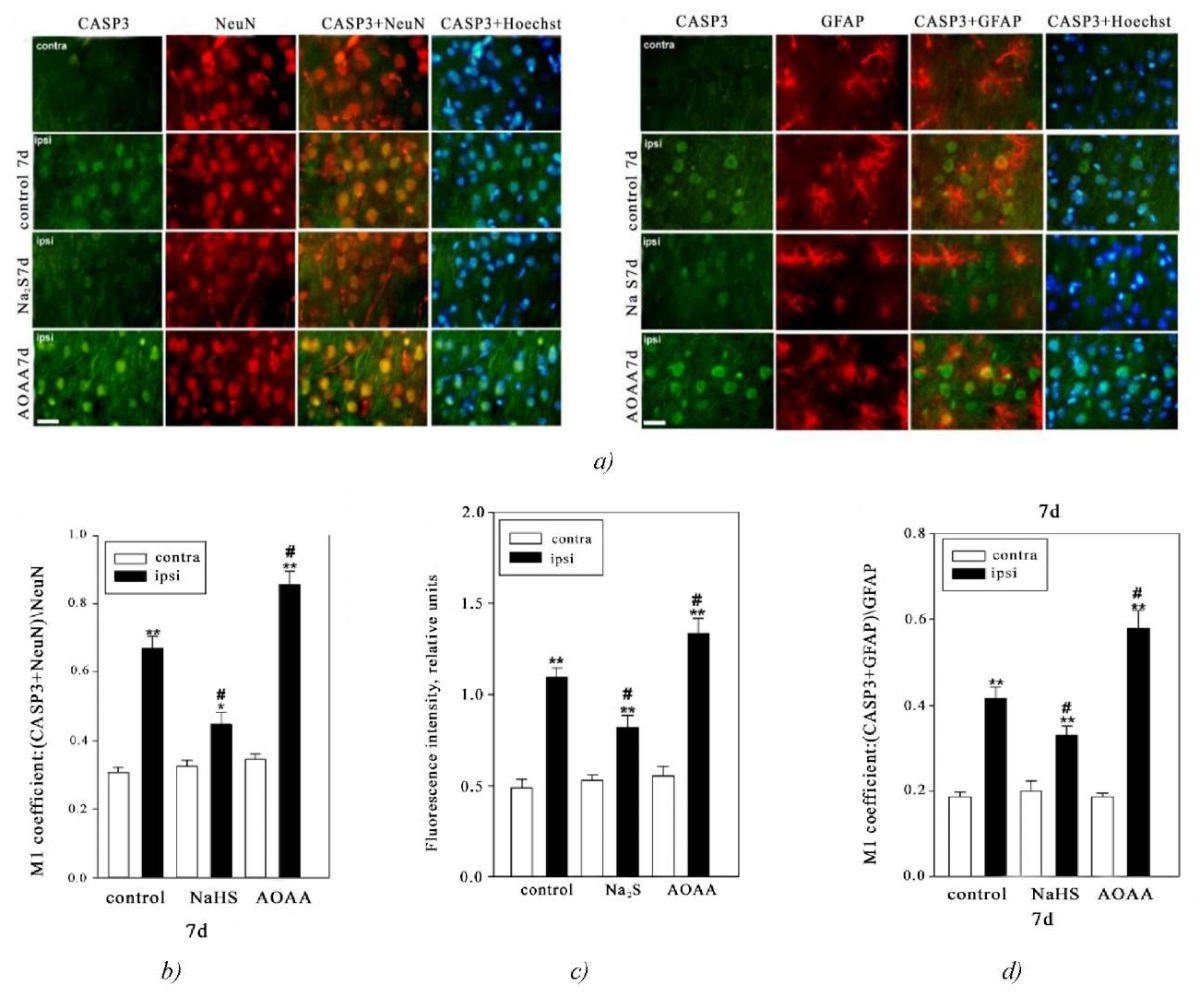
Fig. 1. Immunofluorescence microscopy:
a — expression of caspase-3 (Casp3, green fluorescence) in neurons and astrocytes in the brain of mice from the control group and experimental groups 7 days after injury. Scale bar: 20 μm. NeuN is a marker of neuronal nuclei (red fluorescence); NeuN+Casp3 and Hoechst+Casp3 — colocalization. GFAP is a marker of astrocytes (red fluorescence); GFAP+Casp3 and Hoechst+Casp3 — colocalization. Hoechst fluorescence is Hoechst 33342 (blue fluorescence) visualizing the nuclei of all cells
b — M1 colocalization coefficient of caspase-3 and NeuN in contralateral and ipsilateral neurons in mice from the control group and experimental groups 7 days after injury
c — correlation of the Mean Fluorescence Intensity of caspase-3 in the cytoplasm of neurons of the contralateral and ipsilateral cortex in mice from the control group and experimental groups 7 days after injury
d — M1 colocalization coefficient of caspase-3 and GFAP in astrocytes of contralateral and ipsilateral cortex in mice from the control group and experimental groups 7 days after TBI. Contra — contralateral cortex; Ipsi — ipsilateral cortex. One-way ANOVA. M±SEM. n=6
Note: **p<0.05, **p<0.01 — ipsilateral cortex relative to the contralateral cortex of one animal; #p<0.05 — ipsilateral cortex of mice from the experimental group compared to the ipsilateral cortex of mice from the control group during the same period of time after injury
Herewith, the expression of caspase-3 in neurons of the ipsilateral and contralateral cerebral cortex in mice from the control group and experimental groups differs significantly. This is confirmed by the M1 coefficient values reflecting the degree of colocalization of NeuN and the target protein, namely, caspase-3. In the contralateral cortex, the level of caspase-3 was insignificant in all groups throughout all time intervals after the simulation of TBI. However, traumatic impact led to the rapid increase of caspase-3 expression in neurons of the damaged brain area relative to the contralateral area by 2.2 times 7 days after TBI (p<0.01). The use of Na₂S led to a decrease of caspase-3 level by 32% in the ipsilateral cortex relative to the ipsilateral hemisphere of the animals from the control group (p<0.05) (Fig. 1 b).
The use of the inhibitor of CBS had the opposite effect. Thus, 7 days after the injury, in the damaged neurons in the brain of animals that were injected with AOAA the M1 coefficient value of caspase-3 increased by 31% (p<0.05) relative to the damaged cells in the control group. Also, the expression of caspase-3 increased by 2.5 times (p<0.01) in neurons of ipsilateral hemisphere in the group of animals injected with AOAA relative to the neurons of the opposite hemisphere in the same animal (Fig. 1 b).
Analysis of caspase-3 fluorescence in the cytoplasm of neurons also showed that the level of this enzyme increased in the damaged nerve cells. The fluorescence intensity in the cytoplasm of neurons in animals from the control group and in experimental animals administered Na₂S and AOAA increased by 2.7 times (p<0.01), by 56% (p<0.01), and by 2.4 times (p<0.01), respectively, relative to the contralateral cortex. At the same time, 7 days after TBI, the level of caspase-3 decreased in the ipsilateral cortex of animals administered Na₂S and increased in the group of animals administered AOAA relative to the ipsilateral cortex of animals from the control group by 26% (p<0.05) and 21% (p<0.05), respectively. (Fig. 1 c).
Next, we examined the level of caspase-3 in astrocytes, which were identified using the specific marker GFAP. The colocalization analysis allowed us to establish the expression of caspase-3 in this type of glial cells after TBI. Moreover, the donor Na₂S and the inhibitor AOAA had opposite effect on the dynamics of caspase-3 expression in astrocytes after TBI (Fig. 1 d). Thus, it was shown that the level of this enzyme increased in the ipsilateral cortex of animals from the control group and experimental groups administered Na₂S and AOAA relative to the contralateral cortex by 2.2 times (p<0.01), 65% (p<0.01), and by 3 times (p<0.01), respectively. Also, 7 days after TBI, the level of caspase-3 decreased in the ipsilateral cortex of animals administered Na₂S and increased in the group of animals administered AOAA, relative to the ipsilateral cortex of animals from the control group, by 19% (p<0.05) and by 41% (p<0.05), respectively. (Fig. 1 c).
The fluorescence microscopy data confirmed the results obtained during the Western blot analysis of the total fraction of the brain nervous tissue of animals from the control group and experimental groups administered Na₂S and AOAA (Fig. 2).
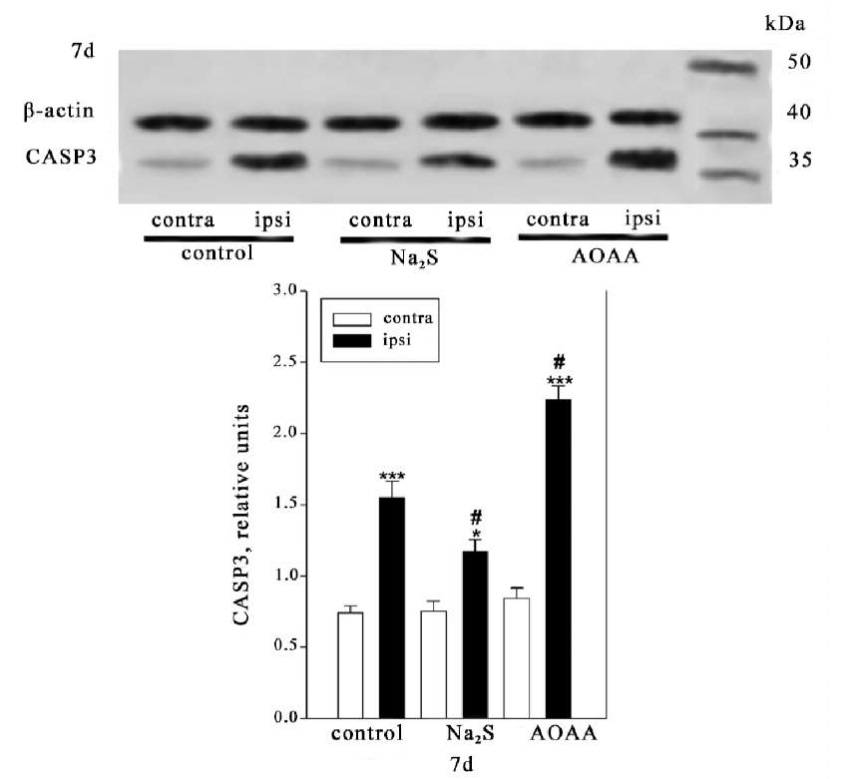
Fig. 2. Western blotting. Caspase-3 expression in the contralateral and ipsilateral cortex of animals from the control group and experimental groups 7 days after injury. One-way ANOVA. M±SEM. n=6.
Note: *p<0.05, ***p<0.001 — ipsilateral cortex relative to contralateral cortex of one animal; #p<0.05 — ipsilateral cortex of animals from the experimental group relative to ipsilateral cortex of animals from control group during the same period of time after injury
It was shown that 7 days after TBI, caspase-3 expression in the ipsilateral cortex of animals from the control group and experimental groups administered Na₂S and AOAA increased relative to the contralateral cortex by 2 times (p<0.001), by 36% (p<0.05), and almost by 2.6 times (p<0.001), respectively. At the same time, caspase-3 expression in the ipsilateral cortex of animals administered Na₂S and AOAA relative to the ipsilateral cortex of animals from the control group decreased by 24% (p<0.05) and increased by 44% (p<0.05), respectively (Fig. 2).
Summarizing the obtained results, based on the immunofluorescence microscopy, it can be concluded that caspase-3 colocalizes with NeuN (neuronal nuclear antigen) and GFAP (astrocyte marker) in neurons and glial cells. In the control group and experimental groups, differences in caspase-3 expression were observed between the ipsilateral (injured) and contralateral (uninjured) cerebral cortex. Injury led to a significant increase of caspase-3 expression in neurons and glial cells in the injured area 7 days after TBI, which indicated activation of apoptosis.
The use of the donor Na₂S decreased the level of caspase-3 in neurons of the ipsilateral cortex, and the inhibitor AOAA caused the opposite effect, increasing the expression of caspase-3. Similar results were obtained when studying caspase-3 in astrocytes, where increased expression was observed in the group administered AOAA and decreased — in the group administered Na₂S.
Western blot analysis of the total fraction of the brain nervous tissue of animals from the control group and experimental groups confirmed the results of immunofluorescence microscopy. Administering Na₂S decreased the expression of caspase-3 in the damaged area, while AOAA fostered its increase. These data indicate an important role of H₂S in the regulation of apoptosis in neurons and glial cells after TBI.
Discussion and Conclusion. Currently, H₂S is known as a powerful regulator of the apoptosis in neurotrauma. H₂S-dependent mechanisms of cell death regulation have been studied in many experimental models using different donors of H₂S and inhibitors of enzymes responsible for H₂S synthesis. However, the role of H₂S-dependent signaling mechanisms in regulating the expression and localization of caspase-3 in neurons and glial cells in TBI has not been sufficiently studied.
In our study, it was shown that H₂S is an important link in control of the caspase-3 level in brain cells in the settings of TBI-simulating induced mechanical injury. The intensity of cell death during TBI-induced secondary injury directly correlates with the level of pro-apoptotic proteins. Caspase-3 is one of the key apoptotic proteins. The increased level of this proteolytic enzyme indicates the initiation of processes leading to cell death. Na₂S — the fast donor of hydrogen sulfide, and AOAA — the traditional inhibitor of the key enzyme of H₂S synthesis in nervous tissue, used in our research, had opposite effects on the expression of this protein. The initial level of caspase-3 in the undamaged neurons and glial cells in the brain was low. However, 7 days after TBI, the level of caspase-3 significantly increased, especially in neurons, as well as in glial cells. The use of Na₂S made it possible to decrease the expression of this enzyme. The opposite effect was observed when CBS was inhibited by AOAA.
H₂S regulates caspase-3 level through a variety of molecular mechanisms, many of which are related to its ability to affect the anti-apoptotic and antioxidant systems in cells. One of the key pathways is increasing expression of Bcl-2 family proteins [14], which inhibit the activation of caspases, including caspase-3 [15]. Bcl-2 interacts with pro-apoptotic effector proteins such as Bax and Bak and prevents the release of cytochrome c from mitochondria. This is an important step in the apoptotic process, as Cyt c is involved in formation of the Apaf-1 apoptosome complex [16], which activates caspases and initiates cell death. H₂S, by increasing the level of Bcl-2, stabilizes mitochondrial membranes and prevents apoptosis
Additionally, H₂S acts as a potent antioxidant efficiently decreasing the level of reactive oxygen species (ROS), which play an important role in the induction of apoptosis through the activation of caspases [4][5][17][18]. An increase in the level of ROS causes mitochondrial damage that leads to the activation of caspases and subsequent cell death. However, reducing properties of H₂S can directly neutralize ROS by decreasing their oxidative potential. This decreases the cellular stress, including stress on mitochondria, and prevents the initiation of the apoptotic cascade. Moreover, H₂S can activate a number of antioxidant enzymes, such as superoxide dismutase and catalase, protecting cells from excessive levels of ROS [4][19].
H₂S can also directly interact with the caspase-3 enzyme. It should be noted that caspase-3 contains an essential cysteine residue that can become a target for modification via the persulfidation process, in which H₂S adds sulfur to the thiol group of cysteine. Persulfidation of cysteine 163 in the active site of caspase-3 leads to inhibition of its proteolytic activity, which prevents the cleavage of caspase-3 substrates and, accordingly, slows down or stops apoptosis [20]. This indicates that H₂S can have a dual effect on apoptosis: indirect — through the regulation of anti-apoptotic and antioxidant pathways, and direct — by inhibiting caspase activation.
Additionally, H₂S can modulate other signaling pathways related to cell death. For example, it can activate Nrf2 (nuclear factor erythroid type 2) signaling pathway, which is a key regulator of cellular antioxidant defence mechanism. Activation of Nrf2 leads to the transcription of multiple genes responsible for antioxidant and cytoprotective functions, thereby decreasing the oxidative stress and protecting cells from apoptotic damage [21][22].
It should also be noted that H₂S can interact with other gasotransmitters, such as nitric oxide (NO) and carbon monoxide (CO), forming the complex signaling networks that regulate cell survival [23]. Synergistic or antagonistic interaction of H₂S with NO and CO can affect various stages of apoptosis, including activation or inhibition of caspases. Thus, in our study, we examined in detail the expression and localization of caspase-3 in neurons and astrocytes in the brain in TBI. The results obtained have both fundamental and practical importance. Data about the role of H₂S in regulation of the key pro-apoptotic protein caspase-3 expand our understanding of the intracellular signaling processes of nervous system cell survival and death, whereas AOAA and Na₂S can become the basis for the development of clinically efficient neuroprotective agents.
References
1. Maas AIR, Menon DK, Manley GT, Abrams M, Åkerlund C, Andelic N, et al. Traumatic Brain Injury: Progress and Challenges in Prevention, Clinical Care, and Research. Lancet Neurology. 2022;21(12):1004–1060. https://doi.org/10.1016/S1474-4422(22)00309-X
2. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Medical Clinics of North America. 2020;104(2):213–238. https://doi.org/10.1016/j.mcna.2019.11.001
3. Ladak AA, Enam SA, Ibrahim MT. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurgery. 2019;131:126–132. https://doi.org/10.1016/j.wneu.2019.07.039
4. Rodkin S, Nwosu C, Sannikov A, Raevskaya M, Tushev A, Vasilieva I, et al. The Role of Hydrogen Sulfide in Regulation of Cell Death Following Neurotrauma and Related Neurodegenerative and Psychiatric Diseases. International Journal of Molecular Sciences. 2023;24(13):10742. https://doi.org/10.3390/ijms241310742
5. Rodkin S, Nwosu C, Sannikov A, Tyurin A, Chulkov VS, Raevskaya M, et al. The Role of Gasotransmitter-Dependent Signaling Mechanisms in Apoptotic Cell Death in Cardiovascular, Rheumatic, Kidney, and Neurodegenerative Diseases and Mental Disorders. International Journal of Molecular Sciences. 2023;24(7):6014. https://doi.org/10.3390/ijms24076014
6. Sun J, Li X, Gu X, Du H, Zhang G, Wu J, et al. Neuroprotective Effect of Hydrogen Sulfide against Glutamate-Induced Oxidative Stress Is Mediated via the p53/Glutaminase 2 Pathway after Traumatic Brain Injury. Aging. 2021;13(5):7180–7189. https://doi.org/10.18632/aging.202575
7. Zhang J, Zhang S, Shan H, Zhang M. Biologic Effect of Hydrogen Sulfide and Its Role in Traumatic Brain Injury. Oxidative Medicine and Cellular Longevity. 2020;2020(1):7301615. https://doi.org/10.1155/2020/7301615
8. Chen D, Fang Y-L, Zhang L-L, Niu X-Y, Sun X-R, Niu X-Z, et al. Hydrogen Sulfide Ameliorates Isoflurane-Induced Cognitive Impairment in Mice: Implication of Caspase-3 Activation. Tropical Journal of Pharmaceutical Research. 2020;19(4):773–780. https://doi.org/10.4314/tjpr.v19i4.14
9. Yang G, Sun X, Wang R. Hydrogen Sulfide‐Induced Apoptosis of Human Aorta Smooth Muscle Cells via the Activation of Mitogen‐Activated Protein Kinases and Caspase‐3. FASEB Journal. 2004;18(14):1782–1784. https://doi.org/10.1096/fj.04-2279fje
10. Kobayashi C, Yaegaki K, Calenic B, Ishkitiev N, Imai T, Ii H, et al. Hydrogen Sulfide Causes Apoptosis in Human Pulp Stem Cells. Journal of Endodontics. 2011;37(4):479–484. https://doi.org/10.1016/j.joen.2011.01.017
11. Ryazantseva NV, Novitsky VV, Starikova EG, Kleptsova LA, Jakushina VD, Kaigorodova EV. Role of Hydrogen Sulfide in the Regulation of Cell Apoptosis. Bulletin of Experimental Biology and Medicine. 2011;151:702–704. https://doi.org/10.1007/s10517-011-1420-y
12. Rodkin S, Nwosu C, Raevskaya M, Khanukaev M, Bekova K, Vasilieva I, et al. The Role of Hydrogen Sulfide in the Localization and Expression of p53 and Cell Death in the Nervous Tissue in Traumatic Brain Injury and Axotomy. International Journal of Molecular Sciences. 2023;24(21):15708. https://doi.org/10.3390/ijms242115708
13. Fletcher PA, Scriven DRL, Schulson MN, Moore EDW. Multi-Image Colocalization and Its Statistical Significance. Biophysical Journal. 2010;99(6):1996–2005. https://doi.org/10.1016/j.bpj.2010.07.006
14. Zhang L-M, Jiang C-X, Liu D-W. Hydrogen Sulfide Attenuates Neuronal Injury Induced by Vascular Dementia Via Inhibiting Apoptosis in Rats. Neurochemical Research. 2009;34:1984–1992. https://doi.org/10.1007/s11064-009-0006-9
15. Mooney SM, Miller MW. Expression of Bcl-2, Bax, and Caspase-3 in the Brain of the Developing Rat. Developmental Brain Research. 2000;123(2):103–117. https://doi.org/10.1016/S0165-3806(00)00081-X
16. Alam M, Alam S, Shamsi A, Adnan M, Elasbali AM, Al-Soud WA, et al. Bax/Bcl-2 Cascade Is Regulated by the EGFR Pathway: Therapeutic Targeting of Non-Small Cell Lung Cancer. Frontiers in Oncology. 2022;12:869672. https://doi.org/10.3389/fonc.2022.869672
17. Luo Y, Yang X, Zhao S, Wei C, Yin Y, Liu T, et al. Hydrogen Sulfide Prevents OGD/R-Induced Apoptosis via Improving Mitochondrial Dysfunction and Suppressing an ROS-Mediated Caspase-3 Pathway in Cortical Neurons. Neurochemistry International. 2013;63(8):826–831. https://doi.org/10.1016/j.neuint.2013.06.004
18. Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid Reactive Oxygen Species (ROS) Generation Induced by Curcumin Leads to Caspase-Dependent and -Independent Apoptosis in L929 Cells. Free Radical Biology and Medicine. 2008;45(10):1403–1412. https://doi.org/10.1016/j.freeradbiomed.2008.08.014
19. Rodkin SV, Nwosu CD. Role of Nitric Oxide and Hydrogen Sulfide in Neuronal and Glial Cell Death in Neurodegenerative Processes. Biologičeskie membrany. 2023;40(5):306–327. https://doi.org/10.31857/S0233475523050067
20. Ye X, Li Y, Lv B, Qiu B, Zhang S, Peng H, et al.. Endogenous Hydrogen Sulfide Persulfidates Caspase-3 at Cysteine 163 to Inhibit Doxorubicin-Induced Cardiomyocyte Apoptosis. Oxidative Medicine and Cellular Longevity. 2022;(1):6153772. https://doi.org/10.1155/2022/6153772
21. Corsello T, Komaravelli N, Casola A. Role of Hydrogen Sulfide in NRF2and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants. 2018;7(10):129. https://doi.org/10.3390/antiox7100129
22. Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, et al. Hydrogen Sulfide Protects Against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxidants and Redox Signaling. 2013;18(15):1906–1919. https://doi.org/10.1089/ars.2012.4645
23. Huang Y-Q, Jin H-F, Zhang H, Tang C-S, Du J-B. Interaction among Hydrogen Sulfide and Other Gasotransmitters in Mammalian Physiology and Pathophysiology. In: Zhu YC. (Ed.) Advances in Hydrogen Sulfide Biology. Advances in Experimental Medicine and Biology. Vol 1315. Singapore: Springer; 2021. P. 205–236. https://doi.org/10.1007/978981-16-0991-6_9
About the Authors
S. V. RodkinRussian Federation
Stanislav V. Rodkin - Cand.Sci. (Biology), Associate Professor of the Bioengineering Department, Head of the Laboratory “Digital Medical Imaging Using the Basic Model”.
1, Gagarin Sq., Rostov-onDon, 344003
E. Yu. Kirichenko
Russian Federation
Evgeniya Yu. Kirichenko - Dr.Sci.(Biology), Professor, Head of the Bioengineering Department.
1, Gagarin Sq., Rostov-on-Don, 344003
The present study is the first one to demonstrate the effect of hydrogen sulfide on caspase-3 in traumatic brain injury. The chemical compound H₂S reduced the level of caspase-3, thus, affected the neuroinflammatory response. An experiment with an inhibitor of H₂S synthesis, AOAA, showed an inverse increase in the expression of this enzyme. The results confirm the importance of H₂S in the regulation of apoptotic cell death in the brain after injury. The study opens up prospects for the development of new neuroprotective agents.
Review
For citations:
Rodkin S.V., Kirichenko E.Yu. H₂S-Dependent Mechanisms of Caspase-3 Expression and Localization in Brain Cells of Mice with Traumatic Brain Injury. Russian Journal of Veterinary Pathology. 2025;24(2):19-28. https://doi.org/10.23947/2949-4826-2025-24-2-19-28. EDN: HPMRMX