Scroll to:
Preclinical Trials in White Mice and Rabbits of the Improved “Associated Inactivated Emulsion Vaccine against Infectious Bovine Rhinotracheitis (IBR), Bovine Viral Diarrhea/Mucosal Disease (BVD), Bovine Parainfluenza Virus 3 (BPIV-3) and Chlamydia in Cattle”
https://doi.org/10.23947/2949-4826-2025-24-3-53-64
Abstract
Introduction. Infectious respiratory and intestinal diseases in cattle are multifactorial diseases, which explains the associated viral or viral-bacterial etiology of the infection among productive livestock. Combating associated respiratory and intestinal infections in cattle is carried in the vast majority of countries in the world and requires efficient measures and medications. The aim of the present research is conducting the preclinical trials in white mice and rabbits of the experimental series of the “Associated inactivated emulsion vaccine against IBR, BVD, BPIV-3 and chlamydia in cattle” improved by expanding the chlamydial antigen spectrum.
Materials and Methods. The trials were conducted in the Animal Viral Disease Laboratory of the Federal Center for Toxicological, Radiation, and Biological Safety (Kazan) from February to November 2024. Two versions of the biopharmaceutical were produced: a standard associated vaccine and an experimental vaccine with the AMK-16 and MZ-89 strains added to the chlamydial antigen. White mice and rabbits served as laboratory animals. The vaccines were evaluated for sterility, safety, tolerability, antigenic activity, impact on antiviral humoral immunity and immunogenicity.
Results. Both versions of the associated vaccine had proved to be sterile, harmless, and well-tolerated by laboratory animals. Changing the chlamydial antigen composition of the associated vaccine did not have an adverse effect on the development of antiviral humoral immunity in laboratory animals. The level of specific anti-chlamydial antibodies in rabbits vaccinated with the improved vaccine was higher than in the group of rabbits vaccinated with the standard technology vaccine. The protection index in the group of white mice vaccinated with the improved vaccine was 1.3 times higher than that with the standard vaccine.
Discussion and Conclusion. Based on the data obtained, it can be concluded that the improved associated vaccine, the same as the standard one, is well tolerated by laboratory animals. Expansion of the chlamydial antigen spectrum of the “Associated vaccine against IBR, BVD, BPIV-3 and Chlamydia in cattle” by adding additional strains did not have any negative effect on the development of antiviral humoral immunity in laboratory animals, but on the contrary had stimulated the development of a humoral response to the chlamydial antigen, thus, boosting the vaccine immunogenicity by 1.3 times.
Keywords
For citations:
Evstifeev V.V., Akbashev I.R., Khusainov F.M., Yakovlev S.I., Khamidullina R.Z., Ivanova S.V. Preclinical Trials in White Mice and Rabbits of the Improved “Associated Inactivated Emulsion Vaccine against Infectious Bovine Rhinotracheitis (IBR), Bovine Viral Diarrhea/Mucosal Disease (BVD), Bovine Parainfluenza Virus 3 (BPIV-3) and Chlamydia in Cattle”. Russian Journal of Veterinary Pathology. 2025;24(3):53-64. https://doi.org/10.23947/2949-4826-2025-24-3-53-64
Introduction. Infectious respiratory and intestinal diseases in young cattle represent a serious problem for livestock farms throughout Russia. The etiology of these pathologies typically involves multiple pathogens simultaneously, representing stable associations of viral and bacterial agents that cause diseases of various manifestations and severity [1–3]. Combating associated respiratory and intestinal infections in cattle is carried out in the livestock industry in the vast majority of countries worldwide [3–5]. An important factor determining the need to improve methods of combating, as well as preventing the respiratory and intestinal infections, is the economic reason: livestock complexes suffer enormous financial losses due to the widespread prevalence of associated infections among livestock [6][7].
Viral pathogens typically cause a primary infection, which initially proceeds in a mild form: at this stage, the immune system is suppressed, which subsequently leads to increased susceptibility to secondary bacterial infections [6][8–10]. However, in some cases, the infectious process development algorithm may be changed, and the pathogens causing the primary infection are represented by the bacteria, which typically are capable of long-term persistence in the infected organism without causing pronounced clinical signs of infection, e.g. chlamydia [11].
Chlamydia are obligate intracellular parasites with a unique two-stage development cycle [12]. These microorganisms are capable of infecting a huge number of animal species. The course of the infectious process in chlamydiosis, even in different populations of the same animal species, is usually not systematic [13], which is due to the evolutionary ability of this type of microorganism to affect various organ systems of animals, causing various clinical signs of the disease (pneumonia, arthritis, conjunctivitis, encephalitis, etc.). In addition, chlamydia can be transmitted from one animal species to another, which also plays a significant role in widespreading this infection among domestic and wild animals [14][15].
The most common viral pathogens in the Russian Federation are those causing infections such as infectious bovine rhinotracheitis (IBR), bovine parainfluenza virus
3 (BPIV-3), bovine viral diarrhea (BVD) [16][17]. Previously, a team of scientists from the Federal Center for Toxicological, Radiation, and Biological Safety (Kazan) developed an “Associated Inactivated Emulsion Vaccine against Infectious Bovine Rhinotracheitis (IBR), Bovine Viral Diarrhea/Mucosal Disease (BVD), Bovine Parainfluenza Virus 3 (BPIV-3) and Chlamydia in Cattle” [17]. The antigenic composition of this biopreparation included one strain each of the IBR, BPIV-3, and BVD viruses, and the Chlamydia psittaci “250” strain isolated from cattle.
As a result of long-term applied and fundamental research into chlamydial infections in animals it was established that different strains of chlamydia of the same species, isolated from the same animal species or from other farm animals with various pathologies, differ from each other antigenically. These differences in the biochemical and genetic structure of chlamydia directly correlate with the immunogenicity of different strains relative to each other [18]. Chlamydia’s capacity for horizontal gene transfer among different species of this pathogen has also been established, allowing some chlamydial strains to contain in their biochemical composition the antigenic epitopes specific to other species of this pathogen [19].
We previously studied the antigenic and immunogenic properties of various chlamydia strains isolated on the territories of different subjects of the Russian Federation [18][20][21]. In the frame of our research, we constructed a new antigenic composition consisting of the three most immunogenic and antigenically distinct chlamydia strains isolated from different animal species. Furthermore, following whole-genome sequencing and subsequent bioinformatics analysis of the nucleotide sequence of the chromosome of one of the strains included in the new antigenic composition, it was established that this strain contains antigenic epitopes specific to two chlamydia species at once—Chlamydia psittaci and Chlamydia abortus [22]. Therefore, using a new chlamydial antigen composition in a new associated vaccine including three chlamydia strains should have good prospects and was implemented.
Thus, the composition of the “Associated vaccine against IBR, BVD, BPIV-3 and Chlamydia in cattle” was supplemented with antigens from two additional chlamydia strains: “AMK-16” (causative agent of arthritis and abortion in goats) and “MZ-89” (causative agent of meningoencephalitis in calves). However, the quantitative ratio of antigens from different pathogens in the vaccine remained unchanged. Preclinical trials in laboratory animals were aimed at determining the effect of the chlamydial antigen composition change on the properties of the “Associated vaccine against IBR, BVD, BPIV-3 and Chlamydia in cattle”.
Materials and Methods. The study was conducted at the Animal Viral Diseases Laboratory of the Federal Center for Toxicological, Radiation, and Biological Safety (Kazan) from February to November 2024.
Strains. The following virus and chlamydia strains were used:
– VK-1 strain of the BVD virus, infectious titer 10-6.87TCID50/ml;
– TK-A (VIEV)-B-2 vaccine strain of the IBR virus of cattle;
– PTK-45/86 reference strain of the BPIV-3 virus of cattle;
– Chlamydia psittaci strain “AMK-16”, isolated from pathological material of an aborted goat;
– Chlamydia psittaci strain “250”, isolated from pathological material of an aborted cow;
– Chlamydia psittaci strain “MZ-89”, isolated from the brain of a calf with the encephalitic form of chlamydial infection;
– Chlamydia psittaci strain “RS-85”, isolated from pathological material from an aborted sow.
Nutrient media. The sterility of the biopreparations was assessed by plating them on the following nutrient media: meat-peptone agar (MPA); meat-peptone broth (MPB); meat-peptone liver broth and Sabouraud medium. The following nutrient media were used for culturing and maintaining the cell culture: synthetic medium 199; Hanks' balanced solution; fetal bovine serum manufactured by the All-Russian Research Institute of Veterinary Medicine; Eagle's medium MEM (Minimum Essential Medium) with glutamine, pH 7.5–7.
Cell cultures. Viruses were cultured using continuous bovine kidney cell culture (MDBK).
Biological models. Chlamydia biomass was obtained by infecting chicken embryos with chlamydia strains into the yolk sac.
Laboratory animals. White mice and rabbits.
During experimental animal experiments, the requirements of Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes were observed. Animals were maintained in optimal conditions and had free access to feed and water.
Reagents and Test Systems. Serological studies were conducted using the following diagnostic test systems:
– “Set of Antigens and Sera for the Serological Diagnosis of Chlamydia in Farm Animals" (ROSS RU.FV01.N00022) manufactured by the Federal Center for Toxicological, Radiation, and Biological Safety of the All-Russian Research Institute of Veterinary Medicine (Kazan);
– “Kit for the Diagnosis of Bovine Parainfluenza-3” (Kursk Biofactory – “BIOK” Firm);
– “Kit for the Detection of Antibodies to the Infectious Bovine Rhinotracheitis Virus by the Enzyme Immunosorbent Assay "IBR-SEROTEST" (LLC “Vetbiokhim”, Moscow);
– “Kit for the Enzyme Immunosorbent Assay of Bovine Viral Diarrhea – Mucosal Disease (BVD) in Cattle” (VIEV, Moscow).
Vaccine composition. Two variants of the associated vaccine with different antigen compositions were prepared for the study. The first (standard) batch of the biopreparation included the following virus strains: BPIV-3 — PTK-45/86", BVD — "VK-1", IBR — "TK-A (VIEV)-V-2", and chlamydia — "250". The second (experimental) batch of the vaccine used similar virus strains, and two more strains — "AMK-16" and "MZ-89" — were added to the chlamydial antigen. The antigens of all strains in the chlamydial antigen were in equal proportions. In both finished vaccine variants, the virus and chlamydia antigens were presented in an equal ratio: BPIV-3, IBR, BVD, and chlamydia in a ratio of 1:1:1:1. Oil-lanolin adjuvant (OLA) was used as an auxiliary component in the production of each vaccine batch. The vaccine emulsion was a water-in-oil system.
Methods. Vaccine sterility was determined in accordance with "OFS 1.2.4.0003.15 General Pharmacopoeia Monograph. Sterility" (Section 2.3) using the direct inoculation method.
The safety of the experimental preparations was determined in accordance with GOST 31926. For each vaccine batch, groups of 15 white mice aged 2 to 3 months and weighing 18 to 25 g were formed. Prepared samples of the test preparations were administered intraperitoneally to the animals in a volume of 0.25 cm³. Subsequently, for 10 days after vaccine administration, daily clinical examinations of the vaccinated animals were conducted to identify sick or dead animals. The vaccine was considered safe if no deterioration in the general condition of the animals or deaths were recorded during the entire observation period.
To evaluate the vaccine tolerance and antigenic activity, 12 rabbits were divided into three groups of four animals each. The first group of animals was vaccinated with the standard vaccine series, while the second group was vaccinated with the experimental vaccine series. Animals in the third group were not vaccinated and served as controls. The animals were administered 0.5 cm3 of the biological preparations intramuscularly into the thigh.
The following parameters were taken into account when assessing the vaccine tolerance of the rabbits:
– General condition of the animals;
– General body temperature after vaccination;
– Presence of a local reaction at the injection site;
– Changes in appetite after vaccination;
– Behavioral reactions.
Vaccine tolerance was assessed during the first 10 days after immunization. The above-mentioned parameters were recorded and recorded daily during clinical examinations of the animals. Body temperature was measured using a mercury thermometer. General condition was assessed visually, focusing on the animals' posture, coat, and gait. Animal appetite was assessed by the presence or absence of food in the feeders after feeding. Vaccine tolerance was assessed by the absence of local or systemic reactions to the administration of the biopreparation.
The antigenic activity of the vaccines was assessed using serological tests. Serum samples were collected systematically from the animals studied over a period of 6 months (on days 30, 60, 90, and 180 post-vaccination). The concentration of antibodies specific to the BPIV-3 virus was determined using the hemagglutination inhibition test (HIT). The level of anti-chlamydial antibodies in the sera of immunized animals was determined using the complement fixation test (CFT). Specific antibodies to the IBR and BVD viruses were determined using an enzyme-linked immunosorbent assay (ELISA).
The ability of the vaccines to induce anti-chlamydial immunity in immunized animals was determined in an acute experiment on white mice (n=180). For each vaccine series, four experimental (eight experimental groups in total) and four control groups of laboratory animals were formed, each containing 15 animals. Animals in the experimental groups were administered the test drugs subcutaneously in a volume of 0.2 cm3. On the 30th day after immunization, animals in all groups were infected with various chlamydia strains. The first experimental groups of mice were infected with the “AMK-16” strain, the second with the “RS-85” strain, the third with the “250” strain, and the fourth with the “MZ-89” strain. The control groups were infected in a similar manner.
- The immunogenicity of biopreparations was assessed by the protection index, which was calculated using the formula:
- (1)
where Х — Protection index; А — number of dead animals in control groups; В — number of dead animals in vaccinated groups.
To confirm the chlamydial etiology of the deaths of infected mice, smears were examined using a light immersion microscope (Nikon Eclipse, Japan). Smears were prepared from the internal organs of dead animals and stained using a modified Stemp method.
Results. Inoculation of two vaccine samples (improved and standard) onto MPA, MPB, MPPPB, and Sabouraud nutrient media revealed no microbial growth on the media during the observation period, confirming the sterility of the test preparations.
Administration of the experimental and standard vaccine samples to white mice did not cause any adverse reactions or negative pathological processes during the observation period, indicating the safety of the preparations.
Observations of immunized and intact rabbits during the vaccine tolerance study revealed that the average body temperature of the animals in the two experimental groups, vaccinated with different variants of the associated vaccine, and the control group remained virtually unchanged throughout the study, ranging from 38.8°C to 39.0°C. All parameters were within normal physiological limits.
Daily clinical examinations of immunized rabbits revealed no pathological conditions. The animals' general condition was satisfactory, their appetite was maintained, and there were no abnormal behavioral reactions. A slight swelling was observed at the injection site of the biological preparations in animals in the experimental groups. This swelling resolved within 10–15 days after vaccination, which is acceptable for immunization with emulsion vaccines.
To determine the effect of the vaccine on the development of post-vaccination humoral immunity, serological studies were conducted to determine the levels of specific antibodies to the antigens used in the experimental and standard vaccines in the blood of rabbits at various times after immunization. The serological results are presented in Table 1.
Table 1 shows that vaccination of rabbits with both biopreparations induced the production of both antiviral and antichlamydial antibodies. On the 30th day after vaccination, the level of immunoglobulins specific to the parainfluenza-3 virus in animals immunized with the standard preparation varied within titers from 1:80 to 1:320. In all rabbits immunized with the experimental preparation, titers of antibodies to the BPI-3 virus were equal to 1:160 at this time point. The average titers of immunoglobulins to the BPI-3 virus in the two groups were equal to a titer of 1:160. The concentration of antiviral antibodies specific to the infectious rhinotracheitis virus in the two groups on the 30th day of the study was within titers from 1:400 to 1:1600.
No significant differences were observed in the development of humoral immunity to the BVD virus. On day 30 of the study, immunoglobulins specific to this virus ranged in titers from 1:800 to 1:1600. A slight difference was observed on day 30 post-vaccination in the development of humoral anti-chlamydial immunity. In the group immunized with the standard preparation, the concentration of complement-fixing immunoglobulins in all animals was at a titer of 1:20. In the group of animals immunized with the experimental preparation, the mean antibody titer was slightly higher, at 1:30.
It should be noted that the lowest concentration of both antiviral and antichlamydial immunoglobulins was detected on the 30th day after vaccination. Over the next two months, an increase in the concentration of antibodies specific to all antigens included in the associated vaccine was observed in the blood serum of immunized animals. Thus, on the 90th day, average antibody titers to the BPIV-3 virus in the HI assay were established at 1:1440 and 1:1600 for the standard and experimental vaccine variants, respectively. Average titers of specific antibodies to the IBR virus in the ELISA during this period were within the titers of 1:5600 for the standard vaccine sample and 1:5200 for the experimental one. On the 90th day after vaccination, average titers of antiviral antibodies to the BVD virus in both groups equaled a titer of 1:3600.
A somewhat different picture was observed when studying blood sera with chlamydial antigen, where a significant difference was found between antibody levels in the two groups of vaccinated rabbits. Thus, the average antibody titer in the serum with chlamydial antigen in the group of animals vaccinated with the standard biopreparation sample was 1:60, while in the group of laboratory animals immunized with the experimental preparation, the average titer was higher, at 1:100. It should be noted that this pattern was also observed at other study time points, on days 60 and 180.
By day 180, the concentration of antiviral and antichlamydial antibodies in the blood serum of immunized animals began to decline in both groups, but was still higher in the experimental group. However, no significant difference was detected between antibody levels to specific antigens across the groups, with the exception of the chlamydial antigen, for which higher antibody levels were detected in the group of animals vaccinated with the experimental variant.
Figures 1, 2, 3, and 4 show the dynamics of average specific antibody titers to viral and chlamydial antigens over the entire observation period (for clarity, the titers are shown separately for each antigen).
Table 1
Testing in rabbits the antigenic activity of the standard and experimental versions of the “Associated vaccine against IBR, BVD, BPIV-3 and Chlamydia in cattle”
|
Antigen / Test |
Vaccine variant |
Animal number |
Antibody titers |
|||
|
30th day |
60th day |
90th day |
180th day |
|||
|
BPIV-3/HI test |
Standard |
1 |
1:80 |
1:1280 |
1:1280 |
1:1280 |
|
2 |
1:80 |
1:640 |
1:1280 |
1:1280 |
||
|
3 |
1:160 |
1:640 |
1:640 |
1:640 |
||
|
4 |
1:320 |
1:640 |
1:2560 |
1:2560 |
||
|
Mean titer |
1:160 |
1:800 |
1:1440 |
1:1440 |
||
|
Experiment |
1 |
1:160 |
1:1280 |
1:1280 |
1:1280 |
|
|
2 |
1:160 |
1:1280 |
1:2560 |
1:2560 |
||
|
3 |
1:160 |
1:640 |
1:1280 |
1:640 |
||
|
4 |
1:160 |
1:320 |
1:1280 |
1:640 |
||
|
Mean titer |
1:160 |
1:880 |
1:1600 |
1:1280 |
||
|
IBR /ELISA |
Standard |
1 |
1:1600 |
1:3200 |
1:6400 |
1:6400 |
|
2 |
1:400 |
1:3200 |
1:3200 |
1:3200 |
||
|
3 |
1:800 |
1:1600 |
1:6400 |
1:3200 |
||
|
4 |
1:800 |
1:1600 |
1:6400 |
1:6400 |
||
|
Mean titer |
1:900 |
1:2400 |
1:5600 |
1:4800 |
||
|
Experiment |
1 |
1:400 |
1:400 |
1:1600 |
1:1600 |
|
|
2 |
1:400 |
1:1600 |
1:6400 |
1:3200 |
||
|
3 |
1:1600 |
1:3200 |
1:6400 |
1:6400 |
||
|
4 |
1:1600 |
1:3200 |
1:6400 |
1:6400 |
||
|
Mean titer |
1:1000 |
1:2100 |
1:5200 |
1:4400 |
||
|
BVD /ELISA
|
Standard |
1 |
1:1600 |
1:6400 |
1:6400 |
1:3200 |
|
2 |
1:1600 |
1:1600 |
1:3200 |
1:6400 |
||
|
3 |
1:800 |
1:800 |
1:1600 |
1:1600 |
||
|
4 |
1:800 |
1:1600 |
1:3200 |
1:1600 |
||
|
Mean titer |
1:1200 |
1:2600 |
1:3600 |
1:3200 |
||
|
Experiment |
1 |
1:800 |
1:1600 |
1:3200 |
1:800 |
|
|
2 |
1:1600 |
1:3200 |
1:3200 |
1:3200 |
||
|
3 |
1:1600 |
1:3200 |
1:6400 |
1:6400 |
||
|
4 |
1:800 |
1:800 |
1:1600 |
1:1600 |
||
|
Mean titer |
1:1200 |
1:2200 |
1:3600 |
1:3000 |
||
|
Chlamydia /CF- test |
Standard |
1 |
1:20 |
1:20 |
1:80 |
1:80 |
|
2 |
1:20 |
1:20 |
1:40 |
1:40 |
||
|
3 |
1:20 |
1:20 |
1:80 |
1:80 |
||
|
4 |
1:20 |
1:40 |
1:40 |
1:40 |
||
|
Mean titer |
1:20 |
1:20 |
1:60 |
1:60 |
||
|
Experiment |
1 |
1:20 |
1:40 |
1:80 |
1:80 |
|
|
2 |
1:40 |
1:80 |
1:160 |
1:80 |
||
|
3 |
1:20 |
1:20 |
1:80 |
1:80 |
||
|
4 |
1:40 |
1:40 |
1:80 |
1:80 |
||
|
Mean titer
|
1:30 |
1:45 |
1:100 |
1:80 |
||
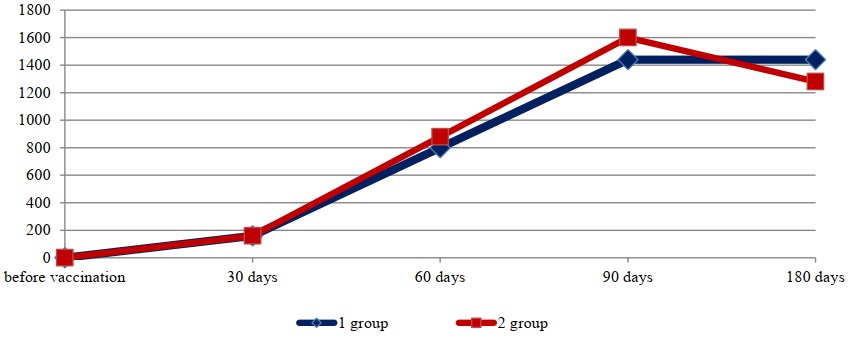
Fig. 1. Mean titers of antiviral antibodies specific to the causative agent of BPIV-3
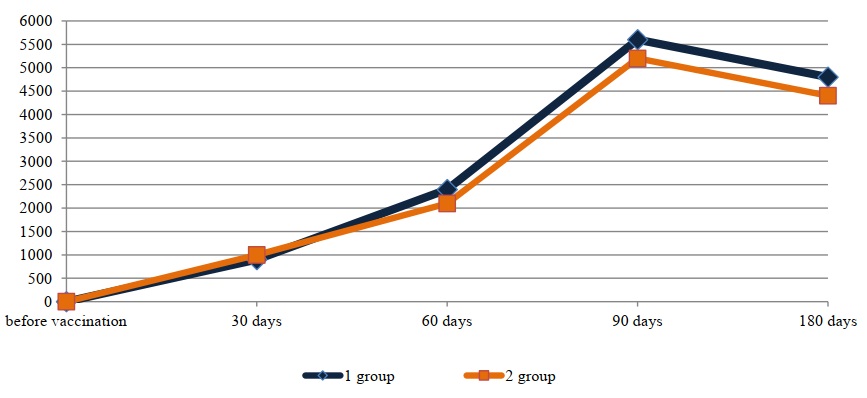
Fig. 2. Mean titers of antiviral antibodies specific to the causative agent of IBR
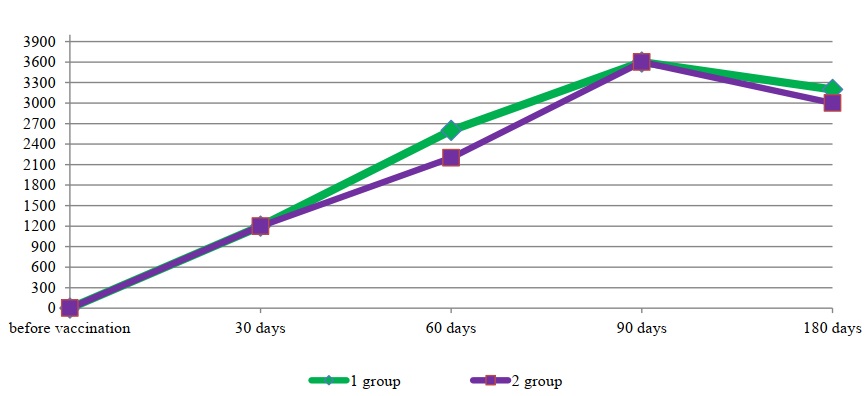
Fig. 3. Mean titers of antiviral antibodies specific to the causative agent of BVD
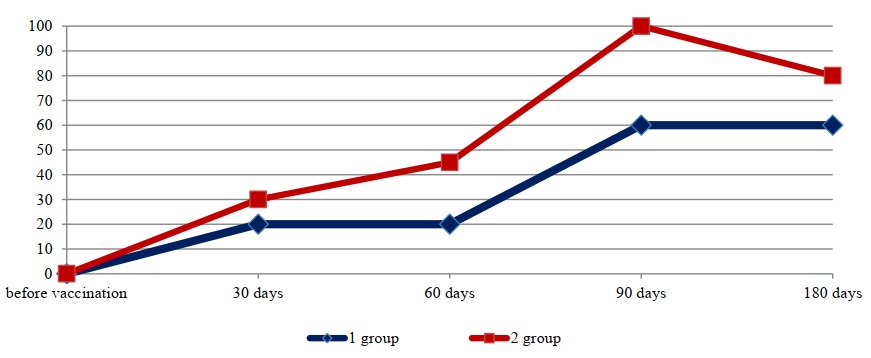
Fig. 4. Average/Mean titers of antichlamydial antibodies
The presented data (Figs. 1, 2, 3, and 4) indicate that altering the chlamydial antigen composition in the associated vaccine did not negatively impact the development of humoral antiviral immunity, but did increase the production of specific antichlamydial antibodies in the blood sera of vaccinated animals.
To determine the effect of the improved vaccine on immunogenicity, an acute experiment was conducted on laboratory white mice. The first and second groups of animals were immunized with the modified vaccine and the standard sample, respectively. All groups of animals were then challenged with four commercial chlamydia strains. The results of these studies are presented in Table 2.
Table 2
Immunogenicity study of standard and experimental vaccine samples in an acute experiment in white mice
|
Batch number of the vaccine for immunization |
Strain for infection |
Number of dead animals |
Surviving animals |
Protection index |
|
Standard |
“250” |
4 |
11 |
3,5 |
|
“PS-85 (РС-85)” |
3 |
12 |
4,3 |
|
|
“АМК-16” |
4 |
11 |
3,75 |
|
|
“MZ-89 (МЗ-89)” |
3 |
12 |
4,7 |
|
|
Total accross groups |
14 |
46 |
4 |
|
|
Experiment |
“250” |
3 |
12 |
4,7 |
|
“PS-85 (РС-85)” |
2 |
13 |
6,5 |
|
|
“АМК-16” |
2 |
13 |
7,5 |
|
|
“MZ-89 (МЗ-89)” |
2 |
13 |
7 |
|
|
Total accross groups |
9 |
51 |
6,2 |
|
|
Control |
“250” |
14 |
1 |
– |
|
“PS-85 (РС-85)” |
13 |
2 |
– |
|
|
“АМК-16” |
15 |
– |
– |
|
|
“MZ-89 (МЗ-89)” |
14 |
1 |
– |
|
|
Total accross groups |
56 |
4 |
- |
|
As shown in Table 2, 46 of the 60 mice vaccinated with the standard vaccine survived infection with four chlamydia strains. The protection indices in the groups of white mice after infection with chlamydia pathogens ranged from 3.5 to 4.7. The average protection index across the four groups of white mice vaccinated with the standard vaccine was 4.
In the groups of mice vaccinated with the improved associated vaccine, the number of surviving animals was significantly higher (51). The protection indices for infection with different chlamydia strains ranged from 4.7 to 7.5. The average protection index across all groups infected with different chlamydia strains and vaccinated with the improved vaccine was 6.2, which is 1.3 times higher than that of the standard vaccine.
In the control groups, after infection with a virulent culture of chlamydia of different strains, only 4 (6.7%) of 60 white mice remained alive.
Discussion and Conclusion. Data obtained during preclinical trials of the improved “Associated inactivated emulsion vaccine against IBR, BVD, BPIV-3 and Chlamydia in cattle”, make it possible to assertain that it is as well tolerated by laboratory animals as the standard vaccine. Altering the chlamydial antigen composition of the vaccine does not adversely affect the development of humoral antiviral immunity in laboratory animals. On the contrary, adding two chlamydia strains to the vaccine stimulates the development of a humoral response to the chlamydial antigen and increases the vaccine’s immunogenicity by 1.3 times compared to the standard vaccine.
References
1. Murray GM, Cassidy JP, Clegg TA, Tratalos JA, McClure J, O’Neill RG, et al. A Retrospective Epidemiological Analysis of Risk Factors for a Primary Necropsy Diagnosis of Bovine Respiratory Disease. Preventive Veterinary Medicine. 2016;132:49–56. https://doi.org/10.1016/j.prevetmed.2016.08.009
2. Callaby R, Toye P, Jennings A, Thumbi SM, Coetzer JA, Conradie Van Wyk IC, et al. Seroprevalence of Respiratory Viral Pathogens of Indigenous Calves in Western Kenya. Research in Veterinary Science. 2016;108:120–124. https://doi:10.1016/j.rvsc.2016.08.010
3. Притыченко А.В., Красочко И.А. Иммуногенность инактивированной ассоциированной вакцины против вирусных респираторных инфекций крупного рогатого скота. В: Материалы Международной научно-практической конференции, посвященной Дню Белорусской науки и 95-летию кафедры эпизоотологии и инфекционных болезней. Витебск; 2023. С. 95–97.
4. Murray GM, O'Neill RG, More SJ, McElroy MC, Earley B, Cassidy JP. Evolving Views on Bovine Respiratory Disease: An Appraisal of Selected Control Measures – Part 2. The Veterinary Journal. 2016;217:78–82. https://doi.org/10.1016/j.tvjl.2016.09.013
5. Bell RL, Turkington HL, Cosby SL. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines. 2021;9(4):337. https://doi.org/10.3390/vaccines9040337
6. Grissett GP, White BJ, Larson, RL. Structured Literature Review of Responses of Cattle to Viral and Bacterial Pathogens Causing Bovine Respiratory Disease Complex. Journal of Veterinary Internal Medicine. 2015;29(3):770–780. https://doi.org/10.1111/jvim.12597
7. Brodersen BW, Kelling CL. Effect of Concurrent Experimentally Induced Bovine Respiratory Syncytial Virus and Bovine Viral Diarrhea Virus Infection on Respiratory Tract and Enteric Diseases in Calves. American Journal of Veterinary Research. 1998;59(11):1423–1430.
8. Woolums AR, Ames TR, Baker JC. The Bronchopneumonias (Respiratory Disease Complex of Cattle, Sheep, and Goats). In: Large Animal Internal Medicine; Louis, MO: Mosby Elsevier; 2015. P. 584–603.
9. Красочко П.А., Красочко П.П., Иващенко И.А. Изучение безвредности различных вариантов ассоциированной вирусно-бактериальной вакцины. В: Материалы Международной научно-практической конференции, посвященной 95-летию со дня рождения доктора ветеринарных наук, профессора Смирновой Нины Ивановны и Дню белорусской науки. Витебская государственная академия ветеринарной медицины: Витебск; 2024. С. 79–84.
10. Глотов А.Г., Глотова Т.И., Нефедченко А.В. Этиологическая структура массовых респираторных болезней молодняка крупного рогатого скота в хозяйствах, занимающихся производством молока. Сибирский вестник сельскохозяйственной науки. 2008;(3(183)):72–78.
11. Федорова В.А., Ляпина А.М., Хижнякова М.А., Зайцев С.С., Салтыков Ю.В., Субботина И.А. и др. Хламидиозы животных и человека. Москва: Наука; 2019. 135 с.
12. Мустафаева Н.А., Сафарова С.А., Джумшудова Ф.А. Бабанлы Л.Т., Мамедова М.A. Хламидиоз сельскохозяйственных животных. Прикаспийский вестник ветеринарии. 2023;(1(2)):24–28.
13. Равилов А.З., Гаффаров Х.З., Равилов Р.Х. Хламидиоз животных. Монография. Казань: Издательство «Фэн» Академии наук Республики Татарстан, 2004. 368 с.
14. Borel N, Sachse K. Zoonotic Transmission of Chlamydia Spp.: Known for 140 Years, but Still Underestimated. In book: Sing A. (Ed.) Zoonoses: Infections Affecting Humans and Animals. Cham: Springer; 2023. P. 1–28. https://doi.org/10.1007/978-3-030-85877-3_53-1
15. Акбашев И.Р. Усовершенстование средств специфической профилактики вирусно-хламидийных инфекций крупного рогатого скота. Дис. канд. ветеринар. наук. Казань; 2021. 129 с.
16. Евстифеев В.В., Гумеров В.Г., Хусаинов Ф.М. Каримуллина И.Г., Акбашев И.Р., Яковлев С.И. и др. Разработка ассоциированной вакцины против ИРТ, ПГ-3, ВД-БС и хламидиоза крупного рогатого скота. Ветеринарный врач. 2020;(6):21–28. https://doi.10.33632/1998-698X.2020-6-21-28
17. Яковлев С.И. Усовершенствование средств специфической профилактики хламидиоза животных. Дис. канд. ветеринар. наук. Москва; 2022. 138 с.
18. Feodorova VA, Zaitsev SS, Khizhnyakova MA, Saltykov YuV, Evstifeev VV, Khusainov FM, et al. Data of de Novo Genome Assembly of the Chlamydia Psittaci Strain Isolated from the Livestock in Volga Region, Russian Federation. Data Brief; 2020;29:105190. https://doi.10.1016/j.dib.2020.105190
19. Евстифеев В.В. Разработка и усовершенствование биологических препаратов для диагностики и специфической профилактики хламидиоза животных. Дис. доктор. биолог. наук. Казань; 2015. 417 с.
20. Zaitsev S, Khizhnyakova M, Saltykov Yu, Evstifeev V, Khusainov F, Ivanova S, et al. Complete Genome Sequence of Chlamydia Psittaci АМК-16, Isolated from a Small Ruminant in the Middle Volga Region, Russia. Microbiology Resource Announcements: 2024;13(5):e0054323. https://doi.10.1128/mra.00543-23
21. Feodorova VA, Zaitsev SS, Lyapina AM, Kichemazova NV, Saltykov YuV, et al. Whole Genome Sequencing Characteristics of Chlamydia Psittaci Caprine AMK-16 Strain, a Promising Killed Whole Cell Veterinary Vaccine Candidate against Chlamydia Infection. PLoS ONE. 2023;18(10):e0293612. (In Russ.) https://doi.10.1371/journal.pone.0293612
About the Authors
V. V. EvstifeevRussian Federation
Vitaly V. Evstifeev, Dr.Sci (Biology), Professor, Chief Research Associate of the Laboratory of Chlamydia Infections; Professor of the Microbiology, Virology, and Immunology Department
2, Nauchny Gorodok, Kazan, Republic of Tatarstan, 420075
35, Sibirsky Tract Str., Kazan, Republic of Tatarstan, 420029
I. R. Akbashev
Russian Federation
Ilgizar R. Akbashev, Cand.Sci (Veterinary Sciences), Research Associate of the Laboratory of Chlamydia Infections
2, Nauchny Gorodok, Kazan, Republic of Tatarstan, 420075
F. M. Khusainov
Russian Federation
Fidail M. Khusainov, Dr.Sci (Veterinary Sciences), Associate Professor, Leading Research Associate of the Labor-atory of Chlamydia Infections
2, Nauchny Gorodok, Kazan, Republic of Tatarstan, 420075
S. I. Yakovlev
Russian Federation
Sergey I. Yakovlev, Cand.Sci (Veterinary Sciences), Research Associate of the Laboratory of Chlamydia Infections
2, Nauchny Gorodok, Kazan, Republic of Tatarstan, 420075
R. Z. Khamidullina
Russian Federation
Razina Z. Khamidullina, Junior Research Associate of the Laboratory of Chlamydia Infections
2, Nauchny Gorodok, Kazan, Republic of Tatarstan, 420075
S V. Ivanova
Russian Federation
Svetlana V. Ivanova, Cand.Sci. (Veterinary Sciences), Leading Research Associate of the Laboratory of Chlamydia Infections
2, Nauchny Gorodok, Kazan, Republic of Tatarstan, 420075
The study represents the first preclinical trials of the improved associated vaccine against IBR, BVD, BPIV-3, and chlamydia in cattle with expanded chlamydial antigen spectrum. Adding the AMK-16 and MZ-89 chlamydia strains did not have negative impact on the development of antiviral immunity, on the contrary, it increased the production of specific antichlamydial antibodies. The protection index in mice vaccinated with the improved product was 1.3 times higher than in mice vaccinated with the standard vaccine. The results confirm that modification of vaccine composition enhances its immunogenicity without deteriorating tolerability. The study demonstrates the potential of the improved vaccine for prophylaxis of the associated respiratory and intestinal infections in cattle.
Review
For citations:
Evstifeev V.V., Akbashev I.R., Khusainov F.M., Yakovlev S.I., Khamidullina R.Z., Ivanova S.V. Preclinical Trials in White Mice and Rabbits of the Improved “Associated Inactivated Emulsion Vaccine against Infectious Bovine Rhinotracheitis (IBR), Bovine Viral Diarrhea/Mucosal Disease (BVD), Bovine Parainfluenza Virus 3 (BPIV-3) and Chlamydia in Cattle”. Russian Journal of Veterinary Pathology. 2025;24(3):53-64. https://doi.org/10.23947/2949-4826-2025-24-3-53-64


























