Scroll to:
Morphological Features and Histogenesis of Canine Nodal Lymphoma
https://doi.org/10.23947/2949-4826-2025-24-3-34-42
Abstract
Introduction. One of the most common types of oncopathologies are lymphoproliferative disorders, in particular, lymphomas. The disorders are characterised by the complicated development mechanisms, multiple etiological factors and their diverse biological features. In addition, the common origin of lymphoid cells makes different types of lymphomas morphologically similar and complicates their diagnostics. The morphological study used to be, currently is and will continue to be a gold standard in the oncological pathology diagnostics. However, the lack of clear morphological criteria and histological classifications of the various types of tumours in animals prevents from adequate diagnostics of oncological diseases in them. The above urges to conduct a thorough study of various morphological types of lymphomas to identify their differential diagnostic features. The aim of the research is to study the morphological features and histogenesis of canine nodal lymphoma to facilitate the diagnostics and develop the efficient targeted therapy protocols.
Materials and Methods. The objects of the study conducted in the network of veterinary laboratories “VETLAB” (Moscow) in the period from January 2024 to January 2025 were the lymph nodes in dogs with the signs of nodal lymphoma. All samples (n = 30) were prepared according to the standard technique of histological sample preparation. Histological preparations stained with hematoxylin and eosin were obtained, and microscopy was performed. A comparative estimation and contrasting against the following morphological criteria were carried out: tumour growth pattern, spread of tumour in the tissue, tumour cell structure, size and location of nuclei, chromatin structure, presence of mitoses, and tumour microenvironment.
Results. By studying the canine nodal lymphoma features by means of microscopy, a number of significant differential diagnostic features were identified enabling to distinguish the following morphological variants of lymphomas: large-cell and small-cell lymphomas, immunoblastic, centroblastic and lymphoblastic lymphomas. Prognostic features included: proliferative index, diffuse growth pattern, cellular microenvironment, and tumour vascularization.
Discussion and Conclusion. The study had identified the main morphological variants and cell types of canine nodal lymphomas. Morphological features and differential diagnostic criteria for microscopic study of lymphomas were determined. The obtained data can be used in the every-day practices of a pathologist for morphological verification of canine lymphomas, and also become the basis for histological classification of tumours of lymphoid tissue in animals.
Keywords
For citations:
Mitrokhina N.V., Sotnikova L.F. Morphological Features and Histogenesis of Canine Nodal Lymphoma. Russian Journal of Veterinary Pathology. 2025;24(3):34-42. https://doi.org/10.23947/2949-4826-2025-24-3-34-42
Introduction. Lymphoma is a widespread malignant neoplasm of haematopoietic and lymphoid tissues in animals and humans. The tumour develops in nodal and extranodal forms, and is not limited to any certain organ, which hinders timely diagnostics of the disease. In human medicine, there are classifications based on comparing clinical, morphological and immunological features resulting in distinguishing numerous variants of T- and B-cell lymphomas [1–3]. All tumours have a common lymphoid cell precursor, similar processes of activation or inhibition of signaling pathways, mutations in key genes, and are characterized by similar genetic disorders and structural abnormalities. All this enables using the new molecular diagnostic methods for clinical purposes to stratify patients into risk groups, develop prognostic and diagnostic models and search for new targets for drug therapy [4]. The common origin of various types of lymphomas complicates their morphological verification. Whereas, lymphoma diagnostic algorithm should begin with morphological examination of tumour cellular composition or its tissue. The objectives of morphological study also include identification and investigation of the prognostic factors; differential diagnostics of indolent and aggressive forms of lymphoma subject to histological transformations; studying the development of aggressive lymphoma during the evolution of an indolent clonal lymphoma [5].
In veterinary medicine, adequate diagnostics of oncological diseases in patients is complicated due to the lack of clear morphological criteria and histological classifications characterizing various tumour types. The development and identification of morphological criteria specific to various lymphoma types will not only improve the quality of differential diagnostics but also enhance the efficacy of subsequent therapeutic interventions. The aim of this study is to present a scientifically based approach to the morphological diagnostics of canine nodal lymphoma, based on the principle of the histogenetic origin of cells.
Materials and Methods. The study was conducted at the network of veterinary laboratories “VETLAB” (Moscow) from January 2024 to January 2025. The objects of the study were 30 lymph nodes in dogs affected by nodal lymphoma. Lymph nodes of various locations were examined; identification of the most common lymphoma locations was not the objective of the present study.
Specimens for morphological examination were selected based on the following criteria: medical history and clinical signs (lymph node enlargement and limited mobility, cachexia, dyspnea); the macroscopically visible signs of lymph node lesion caused by lymphoma (lymph node size greater than 2 cm in diameter, absence of a pattern on lymphoid tissue section, replacement of lymphoid tissue with structureless masses, lumpy surface of lymph nodes, ruptures of capsules); microscopy-based confirmation of nodal lymphoma.
Lymph nodes with macro- and microscopic signs of reactive inflammation, hyperplasia, or metastatic lesions caused by non-lymphoid tumours were excluded from the study. Macroscopic signs of reactive inflammation and hyperplasia in a lymph node include: lymph node size up to 2 cm, preservation of the pattern on the section, smooth lymph node surface and intact capsule. Macroscopic signs of metastatic lesions are as follows: partially preserved pattern on the section, limited tumour foci visible upon the section, and lumpy surface of a lymph node (in cases of multiple metastases).
For the study, a longitudinal incision was made across a lymph node, and up to 0.5 cm diameter section was removed from the tissue core and sent for examination [6].
Histological processing was performed according to the generally accepted protocol: 96% alcohol for 6 hours, xylene for 3 hours, paraffin for 3 hours [7][8]. The specimens were embedded in a paraffin block using a Kedee KD-BMII automatic embedding system (Kedee, Russia). Microtomy of a paraffin block was performed using a Rotary 3004 M semiautomatic microtome (Kedde, Russia). Histological sections were cut at a thickness of 4 μm and fixed on glass slides.
The preparations were stained with hematoxylin and eosin according to the generally accepted protocol: xylene for 10 min, 96% alcohol for 15 min, Mayer’s hematoxylin for 20 min, hydrochloric acid for 10 sec, distilled water for 10 min, eosin solution for 10 min [9], using a Shandon Varistain 24–4 autostainer (Leica, Germany).
Microscopy was performed using a Microscreen microscope (“Minimed”, Russia) at ×100, 200, and 400 magnification.
The following factors were assessed: tumour growth pattern (diffuse, nodular, etc.); cell-related issues (small or large cells, polymorphic cell composition, anaplastic or
blastic/blastoid cell morphology, presence or absence of multinucleated cell forms, characteristics of nuclei structure); and the presence of reactive and residual components. The study adhered to the 5th edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (2022).
Results. The data we obtained during morphological examination of canine nodal lymphomas are presented in Table 1, which shows that the large-cell lymphoma variant was detected most frequently. All tumours had a diffuse growth pattern.
Large-cell tumours had large, eccentrically arranged nuclei. The condensed chromatin structure resulted in nucleoli being differentiated within the nuclei (Fig. 1). At 400x magnification, 5 to 10 mitoses were detected per field of view.
Table 1
Morphological classification of canine nodal lymphoma variants
|
Morphologic classification of canine nodal lymphomas |
Number of cases |
% |
|
Small-cell lymphoma |
6 |
20 |
|
Large-cell lymphoma |
24 |
80 |
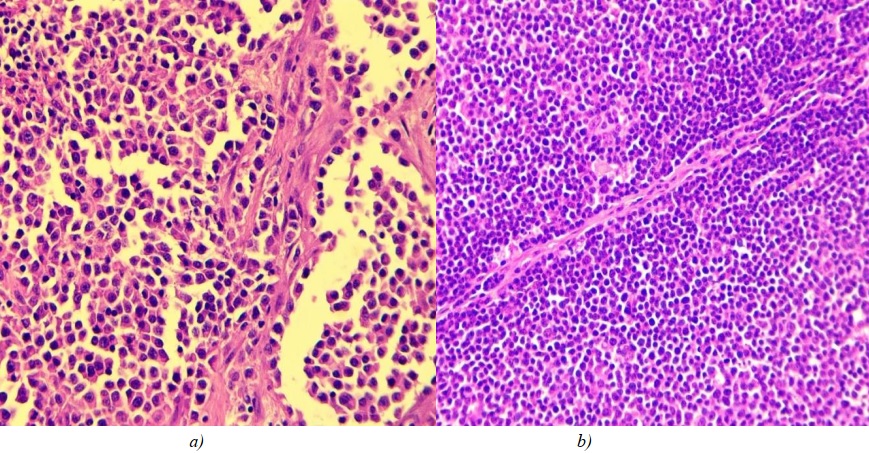
Fig. 1. a — large-cell nodal lymphoma; b — fibrous tissue strand in the center of tumour.
Hematoxylin and eosin staining, ×400 magnification
All large cell nodal lymphomas showed thin fibrous septa, which constitute the tumour stroma. These septa are likely to be the residues of the interfollicular septa of a lymph node (Fig. 1 b).
Nodal lymphomas, composed of small cells, had a number of features: small or medium-sized eccentrically arranged nuclei, tightly packed heterochromatin within the nuclei, and poor differentiation of nucleoli. At ×400 magnification, no more than 5 mitoses per field of view and a large number of apoptotic bodies were detected (Fig. 2).
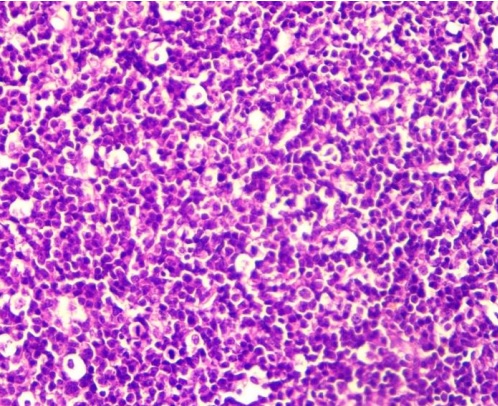
Fig. 2. Small-cell nodal lymphoma. Hematoxylin and eosin staining, ×400 magnification
The results of the study on the histogenetic origin of tumour cells indicate the presence of three cell types: lymphoblastic, immunoblastic and centroblastic (Table 2).
Table 2 shows that the centroblastic cell type of nodal lymphoma is the most common. It is worth noting that immunoblastic and centroblastic types were detected in large-cell variant of lymphoma.
The immunoblastic cell type was characterised by the presence of atypical immunoblasts in the tumour infiltrate — large cells with round, uneven, eccentric nuclei with a single large nucleolus being differentiated, and condensed chromatin loop pattern. The membrane of nucleolus was uneven, often with thin chromatin threads attached to it. The cytoplasmic rim of these cells was quite wide and pale (Fig. 3 a).
The centroblastic cell type was characterized by the presence of atypical centroblastic cells in the tumour infiltrate, which differed drastically from immunoblasts. The morphological features of centroblasts included large, round vesicular nuclei, vesicular chromatin pattern, and
2–3 nucleoli located near the nuclear membrane, as well as moderate amount of cytoplasm (Fig. 3 b).
The lymphoblastic cell type was detected in small-cell tumours and was characterized by the presence of atypical lymphoblasts in the tumour infiltrate—small cells with round nuclei containing compact heterochromatin, undifferentiated nucleolus, uneven nuclear membrane surface and a narrow cytoplasmic rim (Fig. 4 a).
All tumours, regardless of cell type, had poor vascularization. Lymphocytes and plasma cells were present in the microenvironment. Small-cell tumours were infiltrated with macrophages. One case of a large number of eosinophils contained in the microenvironment (Fig. 4 b) was identified.
Table 2
Cell types of canine nodal lymphomas
|
Cell type |
Number of cases |
% |
|
Lymphoblastic |
6 |
20.0 |
|
Immunoblastic |
10 |
33.3 |
|
Centrablastic |
14 |
46.7 |
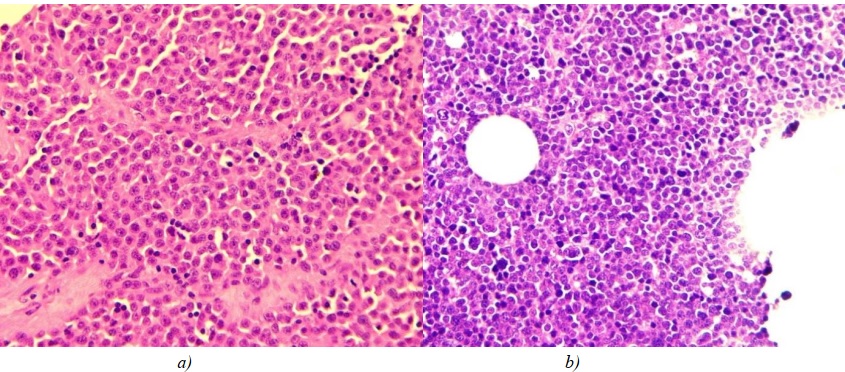
Fig. 3: a — immunoblastic cell type of large-cell nodal lymphoma;
b — centroblastic cell type of large-cell nodal lymphoma. Hematoxylin and eosin staining, ×400 magnification
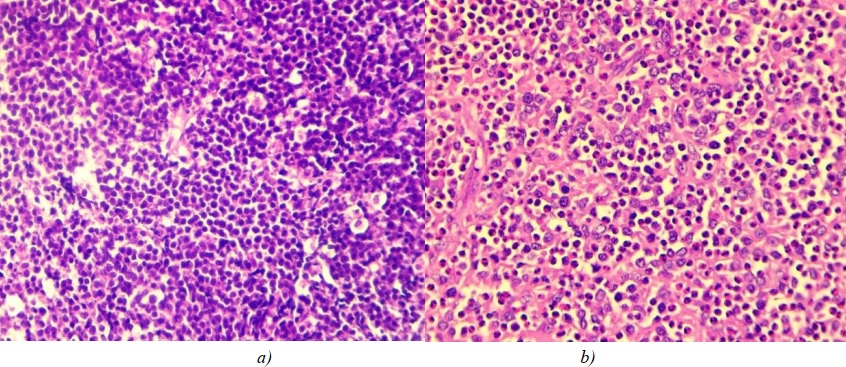
Fig. 4: a — lymphoblastic cell type of small-cell nodal lymphoma; b — immunoblastic cell type of large-cell lymphoma, pronounced tissue infiltration with eosinophils. Stained with hematoxylin and eosin, magnification ×400
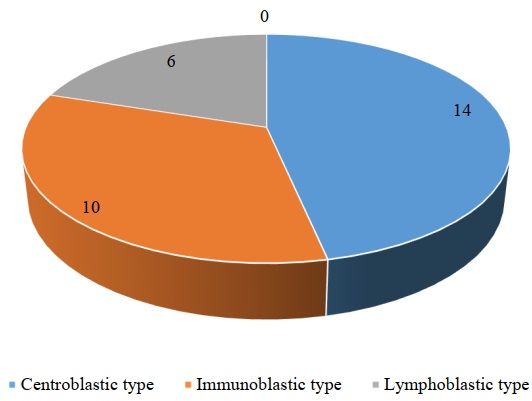
Fig. 5. Results of the study of cell types of canine nodal lymphomas (n = 30)
Discussion and Conclusion. A study of the pathological features of canine nodal lymphoma revealed that all tumours have a diffuse growth pattern. Large-cell and small-cell variants can be identified with regard to morphological classification, and according to histogenetic classification, the tumours are classified into lymphoblastic, centroblastic and immunoblastic. Morphological differential diagnostic criteria include: tumour cell size, size of nuclei, chromatin structure, differentiation of nucleoli, the number of mitoses per field of view and the presence of apoptotic bodies.
According to available literature [10][11], the large-cell variant of lymphoma is most common in humans, which is consistent with the results of our study in dogs. This may be due to the predominance of large B-cells in the body, which are activated during antigenic stimulation.
Analysis of data obtained during the study of tumour cell types shows that the centroblastic cell type is identified most frequently (Fig. 5). It can be assumed that this happens within the process of antigen stimulation, due to B-cells initiating a reaction in the germinal center of the follicle, in which they get transformed into rapidly proliferating centroblasts. Whereas, proliferating cells are more often subject to tumour transformation. In human medicine, diagnostic criteria for lymphoblastic lymphoma include the predominance of atypical lymphoblasts in the tumour substrate and the presence of macrophages in the microenvironment [13–16]. Our data obtained in dogs confirm the above mentioned findings available in the scientific sources.
The centroblastic cell type of lymphoma is characterized by large, round nuclei containing several nucleoli near the nuclear membrane, whereas atypical immunoblasts, which make up the cellular substrate of immunoblastic lymphoma, have large, uneven nuclei containing a single nucleolus. The cytoplasmic rim of atypical immunoblasts is more abundant than that of centroblasts. Similar differential diagnostic features were mentioned by L.G. Babicheva and I.V. Poddubnaya in their description of nodal lymphoma in humans [17].
According to the 5th edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (2022) currently used in human medicine, diffuse large-cell lymphoma has 14 clinical and biological forms [17–20]. These forms have not been studied or identified in animals; therefore, the results of our study may pave the way for the exploration of morphological features and differential diagnostic criteria for a larger number of nosological forms of lymphoid tissue tumours in veterinary pathology. Our study confirms the histogenetic origin of tumour cells, which should be reflected in the histological classification.
References
1. Kovrigina AM, Probatova NA. Hodgkin's Lymphoma and Large Cell Lymphomas. Moscow: Meditsinskoe informatsionnoe agentstvo; 2007; 216. (In Russ.)
2. Ursu D, Gudumac E, Bernic Ja, Tîbîrnă A, Railean S, LUPAN R., et al. Diagnostic Peculiarities of Lymphomas in the Cervical Region in Children. Bulletin of the Academy of Sciences of Moldova. Medical Sciences. 2023;76(2)36-41. https://doi.org/10.52692/1857-0011.2023.2-76.06
3. Kudacheva NA, Bespalova TYu. Clinical and Morphological Diagnosis of the Lymphoma among Dogs. International Research Journal. (In Russ.) 2017;(9–2(63)):14–17. https://doi.org/10.23670/IRJ.2017.63.006
4. Kovrigin AM. Does the Mutation in the Myd88 L265P Gene in Diffuse Large B-Cell Lymphomas Have Independent Diagnostic Significance? Gematologiya i transfuziologiya (Russian Journal of Hematology and Transfusiology). 2024;69(4):499–501. (In Russ.) https://doi.org/10.35754/0234-5730-2024-69-4-499-501
5. Magomedova AU, Kovrigina AM, Mangasarova YaK, Kravchenko SK, Nikulina EE, Obukhova TN, et al. Transformation of Indolent Lymphomas into Diffuse Large B-Cell Lymphoma. Gematologiya i transfuziologiya (Russian Journal of Hematology and Transfusiology).2024;69(1):112-120. (In Russ.) https://doi.org/10.35754/0234-5730-2024-69-1-112-120
6. Yunusova YuR, Plokhova VA, Shuvalova TV, Poletaeva SV, Sukhachev PA, Kirichenko ND. Handbook on Clinical Pathological Anatomy. A Textbook for Students of Higher Educational Medical Institutions. Samara: Polygraphic Association “Standard”; 2020. 121 p. (In Russ.)
7. Asaulenko ZP, Chukhrai SM, Yakovleva AI, Alymova EV, Buchaka AS, Pavlinov GB. The Effect of a Shortened Standard Specimen Preparation Procedure on the Quality of Histological Preparations. In: Collection of Abstracts of the Second Russian Congress of Laboratory Histotechnology with International Participation “Histotechnology”. Moscow; 2024. P. 31–34. (In Russ.)
8. Peshkov MV, Dygalo II. Histological Tissue Processing Procedure, by Using Isopropanol and Mineral Oil. Arkhiv patologii (Russian Journal of Archive of Pathology). 2009;71(3):39–41. (In Russ.)
9. Bezrukov AV, Belanov ME, Borisoglebsky AA, Bukharov GA, Kravtsov PA, Kuznetsov MV, et al. New Family of Automated Slide Stainers. Polyclinic. 2023;(4(1)):28–31. (In Russ.)
10. Kalenik OA. Results of Chemoimmunotherapy in Patients with GCb-Subtype Diffuse B-Cell Non-Hodgkin Lymphoma. Journal of the Grodno State Medical University. 2022;20(6):599-602. (In Russ.) https://doi.org/10.25298/2221-8785-2022-20-6-599-602
11. Vadasz B, Wolniak K, Sukhanova M, Chen YH, Behdad A. Leukemic Presentation of Anaplastic Large Cell Lymphoma: A Diagnostic Challenge Mimicking T-Cell Prolymphocytic Leukemia. AJSP Reviews & Reports. 2022;27(3):119–122. https://doi.org/10.1097/pcr.0000000000000511
12. Rukavitsyn OA (Ed.). Hematology: National Guidelines. 2nd Edition, Revised and Supplemented. Moscow: GEOTAR-Media Publ.; 2024. 916 p. (In Russ.) https://doi.org/10.33029/9704-8188-2-GEM-2024-1-916
13. Lipartia MG, Ashurova DT, Daminova MN. Non-Hodgkin’s Lymphoma in Children. Eurasian Bulletin of Pediatrics. 2022;1(12):39–42. URL: https://goo.su/zko6XF (accessed: 01.09.2025)
14. Lipartia MG, Daminova MN. Optimization of Diagnostics of Anaplastic Large Cell Lymphoma in Children. Journal a New Day in Medicine. 2022;1(39):119–122. URL: https://newdayworldmedicine3d.com/upload_files/journal_article/65b23c7d9dee3.pdf (accessed: 01.09.2025)
15. Krylova YaV, Mikhailova NB, Lepik EE, Lepik KV, Beynarovich AV, Smirnova AG, et al. T-Cell Prolymphocytic Leukemia (T-PLL): A Clinical Case and Review of the Literature. Cellular Therapy and Transplantation. 2023;12(4):43–49. https://doi.org/10.18620/ctt-1866-8836-2023-12-4-43-49
16. Quintanilla-Martinez L, Sander B, Chan JKC, Xerri L, Ott G, Campo E, et al. Indolent Lymphomas in the Pediatric Population: Follicular Lymphoma, IRF4/MUM1+ Lymphoma, Nodal Marginal Zone Lymphoma And Chronic Lymphocytic Leukemia. Virchows Archiv. 2016;468:141–157. https://doi.org/10.1007/s00428-015-1855-z
17. Babicheva LG, Poddubnaya IV. Heterogeneous Diffuse Large B-Cell Lymphoma: Accurate Diagnosis as a Key to Successful Therapy. A review. Journal of Modern Oncology. 2023;25(2):168–177. (In Russ.) https://doi.org/10.26442/18151434.2023.2.202237
18. Igonin YuA, Dolgov OYu, Khavaneva OV. Lymphomas: A tutorial. Cheboksary: Chuvash State University Named after I.N. Ulyanov; 2020. 104 p. (In Russ.)
19. Krivolapov YuA, Leenman EE. Morphological Diagnostics of Lymphomas. St. Petersburg: “Kosta” Publ.; 2006. 208 p. (In Russ.)
20. Baram DV, Asaulenko ZP, Spiridonov IN, Krivolapov YuA. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, 2022 (5th Edition): Lymphoid Tumors. Russian Journal of Archive of Pathology. 2023;85(4):24–31. (In Russ.) https://doi.org/10.17116/patol20238504124
About the Authors
N. V. MitrokhinaRussian Federation
Natalia V. Mitrokhina, Cand.Sci. (Veterinary), Head Physician of the Network
1, Bronnitsky Lane, Moscow, 109202
L. F. Sotnikova
Russian Federation
Larisa F. Sotnikova, Dr.Sci. (Veterinary), Professor, Head of the Department of Diseases of Small Domestic Laboratory and Exotic Animals
Moscow
Supplementary files
In the study, the morphological features and histogenesis of canine nodal lymphoma were systematized for the first time based on the analysis of 30 cases in dogs. Two main morphological tumour variants were identified—large cell (80%) and small cell (20%) lymphomas, as well as three cell types of lymphoma were classified by histogenetic origin into: centroblastic (46.7%), immunoblastic (33.3%), and lymphoblastic (20%). All tumours were attributed with a diffuse growth pattern and poor vascularization. The results confirm the importance of such morphological criteria as cell size, chromatin structure and number of mitoses for differential diagnostics. The study establishes a scientific basis for the histological classification of lymphomas in animals and provides practical tools for improving the diagnostics and developing the targeted therapy in veterinary oncology.
Review
For citations:
Mitrokhina N.V., Sotnikova L.F. Morphological Features and Histogenesis of Canine Nodal Lymphoma. Russian Journal of Veterinary Pathology. 2025;24(3):34-42. https://doi.org/10.23947/2949-4826-2025-24-3-34-42


























